Sinonasal Melanoma: About Two Cases and Literature Review
ABSTRACT
Mucosal malignant melanoma of the nasal cavity and paranasal sinuses is a rare tumor, given their rarity and the absence of randomized trials, the diagnostic and therapeutic management of these tumors is difficult. We report two cases of mucosal malignant melanoma of nasal cavity and through the literature data we review the different aspects of this rare entity. Both patients underwent surgical excision with removal of the tumor. The radiotherapy was delivered with intensity modulated radiation therapy (IMRT) technique. The prognosis remains unfavorable despite therapeutic progress.
KEYWORDS
Melanoma; Sinus; Paranasal; Radiotherapy; IMRT
INTRODUCTION
Malignant melanoma is a malignant tumor of epithelial tissue origin. It has a low incidence rate, often occuring in the skin, but rarely in the mucosa [1,2]. Malignant melanoma of the nasal cavity is very aggressive tumor with a complex treatment and unfavorable prognosis. Its treatment is essentially surgical and is complemented by radiotherapy with modern technique as IMRT.
OBSERVATION 1
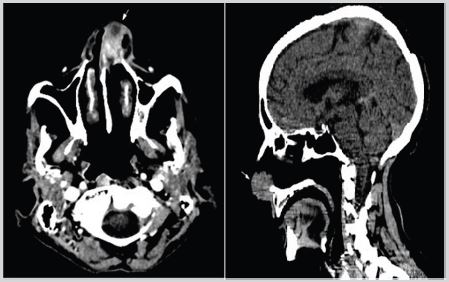
Mrs ND, 72 years old, widow and mother of 7 children, who had no particular pathological antecedents or toxic habits, consulted for a left unilateral nasal obstruction without other associated signs, The patient consulted the otorhinolaryngology department where she underwent a craniofacial CT scan and found a tissue process in the left nasal cavity, developed at the level of the nostril lumen and obstructing it, with irregular contours, isodense, heterogeneously and significantly enhancing after injection of contrast, measuring approximately 17x28mm in diameter. This process infiltrates the anterior portion of the nasal septum and extends downward at the level of the nasal pyramid and towards the homolateral nasolabial fold. It discreetly lyses the anterior internal wall of the left maxillary sinus (Figure 1).
Then the patient was admitted to the operating room, the exploration found a tumor lesion in the left nostril which is completely obstructed by the tumor, adherent to the septum and the nasal floor without cartilage invasion, the patient benefited from a total removal of the tumor. The anatomopathological study of the surgical specimen showed numerous spindle-shaped cells, arranged in tangled bundles, dissociated by more or less thickened fibrous trabeculae, the tumor cells are sometimes strongly pigmented and represent voluminous nuclei, The mitotic index is evaluated at 30 mitoses per 10 CFG.
This tumor proliferation, richly vascularized, destroys the epidermis on the surface and infiltrates the dermis and hypodermis in depth, as well as the nostril cartilage. The breslow index was evaluated at 9 mm in the deepest zone, no vascular emboli or peri-nervous sheathing, the immunohistochemical study showed tumor cells positive for anti- PS100, anti-HMB45 and anti-Melan A, anti-CD34, anti-CK, anti-AMI, anti-desmin, anti- Hcaldesmone were negative in the tumor cells; the histological and IHC aspect of a desmoplastic malignant melanoma, ulcerated, Clark level V was retained.
The case was discussed in a multidisciplinary ENT (ear - nosethroat) oncology consultation meeting and the decision was to perform a recent extension assessment and radiotherapy.
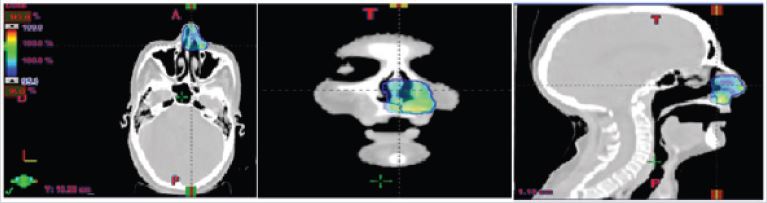
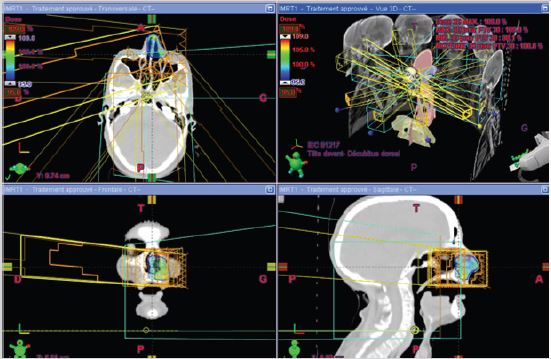
A craniocervical-thoraco-abdomino-pelvic CT scan was performed, which showed no mass syndrome in the left nasal cavity and no suspicious abnormalities on the other floors. The patient received hypofractionated radiotherapy with a total dose of 30 Gy in 5 fractions, two fractions per week, 6 Gy per fraction using the IMRT technique (Figure 2,3). The patient received treatment according to plan and tolerance was good. She was reviewed weekly during treatment by our team. No significant side effects were observed.
OBSERVATION 2
Mr AE, 77 years old, married and father of 10 children, worker as a salesman, diabetic for 6 years, chronic smoker for 30 years, severe 3 years ago. He has as family antecedents a brother deceased by undocumented neoplasia, and a daughter deceased of a leukemia.
The starting of symptoms goes back to 6 months ago, when the patient presented an intermittent bleeding from the right nasal cavity with homolateral nasal blockage, he consulted an ENT specialist, where an endoscopic exploration with biopsy was made. Anatomopathological examination showed an undifferentiated process compatible with an achromic malignant melanoma, with lateral and deep surgical sections difficult to specify.
The patient was investigated with a CT scan of the paranasal sinuses, which revealed a soft tissue lesion in the right nasal cavity that was extending into the right ethmoid sinus with no regional lymphnodes. The magnetic resonance imaging (MRI) showed that the tumor was very close to the floor of the sphenoid sinus and the right frontal sinus with suspicion of involvement of the floor of the orbit. Further staging assessment, which included CT scan of the chest and abdomen, was carried out with no evidence of distant metastasis.
The patient underwent a tumor excision with a right lateral rhinotomy. The surgery removed the maximum tumor bulk from the sino-nasal region with excision of the lacrymo-nasal canal. The histopathology of the surgical specimen was consistent with mucosal malignant achromic melanoma. The margins could not be specified. The lacrymo-nasal canal was not involved by the melanoma. The case was then discussed in a multidisciplinary ENT oncology consultation meeting and the decision was adjuvant radiotherapy.
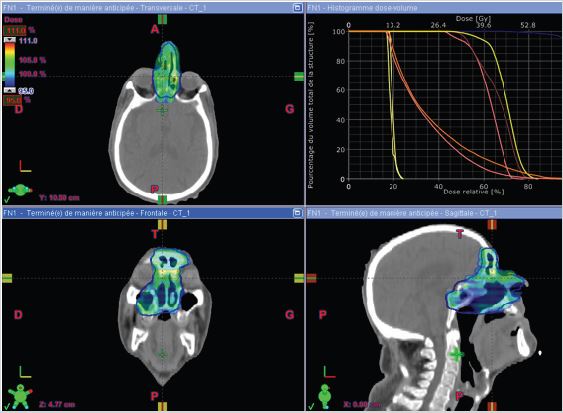
The patient received radiotherapy with a total dose of 66 Gy in 33 fractions, one fractions per day, 2 Gy per fraction using the IMRT technique (Figure 4). The patient received treatment according to plan and tolerance was good. She was reviewed weekly during treatment by our team. No significant side effects were observed. except moderate skin reactions on the irradiated skin and occasional right nasal blockage at the end of treatment. The patient was reviewed after 1 month. Skin reactions almost resolved. The patient was doing well. He received a follow-up CT scan after 4 months which showed no signs of local recurrence, and no secondary localization.
DISCUSSION
Malignant melanoma is a malignant tumor of epithelial tissue origin. It has a low incidence rate, often occuring in the skin, but rarely in the mucosa [1,2]. It represents between 4 and 8% of malignant tumors of the nasal cavity and paranasal sinuses [3]. The age at the time of diagnosis is between 60 and 80 years with a mean age between 65 and 70 years [4].
The lateral wall of the nasal cavity and the nasal septum are common sites for primary nasal mucosal malignant melanoma, accounting for 41.4% and 24.1% of cases, respectively. The study found that the prognosis of patients with melanoma of the nasal septum was better than that of patients with melanoma of the sinuses or nasal lateral wall, which was similar to the findings of Patel et al. [5]. This study suggests that the symptoms of melanoma in the nasal septum manifest at an earlier clinical stage than that of melanoma located at other locations, including the sinuses and nasal lateral wall, which subsequently results in earlier diagnosis and treatment [6].
This diagnosis is difficult because of a double architectural and cytological polymorphism. The diagnosis of certainty is provided by immunohistochemistry techniques that allow the identification of melanocytic differentiation markers and the elimination of another tumor causes [7]. Imaging assessment includes computed tomography (CT) (for bony structures). Magnetic resonance imaging (MRI) is essential for local and regional staging of the tumor [8]. It defines the tumor extension to the orbit and skull base and also shows brain metastases. Distant staging is based on PET/ CT or chest, abdomen and pelvic CT.
The treatment of melanoma of the nasal cavity is essentially surgical, with a large excision of the tumor, ensuring a safety margin of at least 2cm, [9]. The contribution of radiotherapy and chemotherapy is debatable depending on the authors [10]. Postoperative radiotherapy is generally considered for the majority of patients [11,12], although its role remains unclear [13-15]. Several studies have concluded that the addition of radiotherapy to surgical removal provides a local control benefit even for small tumors. A few studies have suggested improved survival in patients receiving postoperative radiotherapy [16,17]. Although most published series have not found this survival benefit, many authors recommend aggressive local treatment with adjuvant or salvage radiotherapy for patients with nasal cavity or nasosinus melanomas [12,17,18]. Hypofractionated radiotherapy is recommended by most authors [14]. Doses are variable, are not well established, and vary according to the series. Some techniques such as intensity modulated radiotherapy or proton therapy allow the optimization of radiotherapy without increasing side effects. Clinical prognostic factors are tumor size, recurrence, and lymph node and distant metastases. Histological predictors of poor prognosis are deep tissue extension, presence of an undifferentiated component greater than 25%, papillary or sarcomatoid architecture, presence of necrosis and vascular emboli [2]. The evolution is often unfavorable. Recurrence is seen in 60% to 80% of cases. The 5-year survival rate varies from 10% to 47% [2,9]. Metastases are frequent and are mostly seen in the lungs, brain and liver. They are rare at the time of diagnosis but are founded in 40 to 50% of cases during the course of the disease.
CONCLUSION
Malignant mucosal melanoma of the nasal cavity has an unfavorable prognosis, Management including surgery and postoperative hypo-fractionated intensity-modulated radiotherapy could contribute to improve overall survival. Immunological therapies (cytokines, interleukins, BCG) deserve randomized studies.
REFERENCES
- Manolidis S, Donald PJ (1997) Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer 80(8): 1373-1386.
- Prasad ML, Busam KJ, Patel SG, Hoshaw-Woodard S, Shah JP et al. (2003) Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch Pathol Lab Med 127(8): 997-1002.
- Mihajlovic M, Mihajlovic S, Jovanovic P, Stefanovic V (2012) Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol 5(8): 739-753.
- Patrick RJ, Fenske NA, Messina JL (2007) Primary mucosal melanoma. J Am Acad Der- amatol 56(5): 828-834.
- Patel SG, Prasad ML, Escrig M, Singh B, Shaha A, et al. (2002) Primary mucosal malignant melanoma of the head and neck. Head Neck 24(3): 247-257.
- Huanin Y, Gang L (2015) Clinical analysis of 29 cases of nasal mucosal malignant melanoma. Oncol Lett 10(2): 1166-1170.
- Saint-Blancard P, Kossowski M (2006) Mélanomes des muqueuses nasosinusiennes. Presse Med 35(1): 1664-1667.
- Erraisse MA, Soussy K, Ali M, Khalid M (2018) Management of sinonasal melanoma: Case report. Int J Nuclear Med Radioactive Subs 1(2): 000107.
- Kharoubi S (2005) Malignant tumors of nasal fossae: anatomoclinic’s study and a new classification: study about 21 cases. Cancer Radiother 9(3): 187-195.
- Prasad ML, Perez OB (2009) Nonsquamous lesions of the nasal cavity, paranasal sinuses, and nasopharynx. In: Gnepp DR (Ed.), Diagnostic surgical pathology the head and neck. Elsevier, USA, pp.111-189.
- Pomar BP, San RCJ, Bouso MM (2007) Sinonasal mucosal melanoma. An Otorrinolaringol Ibero Am 34(4): 349-358.
- Kingdom TT, Kaplan MJ (1995) Mucosal melanoma of the nasal cavity and paranasal sinuses. Head Neck 17(3): 184-189.
- Lee SP, Shimizu KT, Tran LM, Juillard G, Calcaterra TC (1994) Mucosal melanoma of the head and neck: the impact of local control on survival. Laryngoscope 104(2): 121-126.
- Krengli M, Jereczek-Fossa BA, Kaanders JH, Masini L, Beldi D, et al. (2008) What is the role of radiotherapy in the treatment of mucosal melanoma of the head and neck? Crit Rev Oncol Hematol 65(2): 121-128.
- Wada H, Nemoto K, Ogawa Y, Hareyama M, Yoshida H, et al. (2004) A multi-institutional retrospective analysis of external radiotherapy for mucosal melanoma of the head and neck in Northern Japan. Int J Radiat Oncol Biol Phys 59(2): 495-500.
- Lund VJ, Howard DJ, Harding L, Wei WI (1999) Management options and survival in malignant melanoma of the sinonasal mucosa. Laryngoscope 109(2): 208-211.
- Nakashima JP, Viégas CM, Fassizoli AL, Rodrigues M, Chamon LA, et al. (2008) Postoperative adjuvant radiation therapy in the treatment of primary head and neck mucosal melanomas. ORL J Otorhinolaryngol Relat Spec 70(6): 344-351.
- Hyodo M, Sato H, Yamagata T, Hato N, Aritomo H (1996) Sinonasal malignant melanoma; clinical analysis of 14 cases. Practica Oto- Rhino- Laryngologica 2: 121-126.
Article Type
Case Report
Publication history
Received Date: December 06, 2021
Published: December 30, 2021
Address for correspondence
Bouziane Amina, Department of radiation oncology, University hospital Hassan II, Fez, Morocco
Copyright
©2021 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Bouziane A, Bouziane J, Ait EM, Hassani W, Farhan FZ, Alami Z, Bouhafa T. Sinonasal Melanoma: About Two Cases and Literature Review. 2021- 3(6) OAJBS.ID.000369.
Figure 1: Axial and sagittal CT scan slide showing the left nasal tumor.
Figure 2: Axial sagittal and coronal views of the conformity of the prescribed dose to the target volume in blue.
Figure 3: Disposition of multiple IMRT beams on the three plans (axial, sagittal and coronal).
Figure 4: Disposition of multiple IMRT beams on the three plans (axial, sagittal and coronal) and dose-volume histogram curves.