Reliability of Alpha-Defensin 1 Assay in Detecting Periprosthetic Joint Infections: A Pilot Study
ABSTRACT
Introduction: The intraoperative diagnosis of Periprosthetic Joint Infection (PJI) is a surgical dilemma often requiring sampling
of suspicious tissues for frozen section (FS), deep tissue cultures & histo-pathology (HP). Synovial fluid biomarker Alpha defensin-1
(AD-1) provides rapid intraoperative biomarker testing to assist in detecting PJIs. We aim to study the accuracy of Synovasure TM
in detecting periprosthetic joint infections at our institute.
Methods: 42 patients who underwent revision surgery for suspected periprosthetic joint infections were included in our study
following ethics approval. Synovial fluid from these patients were tested with the Synovasure test kit intraoperatively. Results were
compared with Lab results to compare and assess for false positive and negative results.
Results: 6 patients tested positive with 3 true positives and 3 false positives were also. 36 patients tested negative with 1 false
negative and 35 tree negatives.
Conclusion: No perfect intraoperative test for PJI exists however false negative results are rare using Synovasure had a 97%
specificity and 50% sensitivity rate in detecting periprosthetic joint infections at our institution. This system gives rapid results with
fewer false negatives than conventional frozen section testing.
KEYWORDS
Synovasure; Alpha defensin; Periprosthetic joint infection; Revision, Hip, Knee, Arthroplasty
INTRODUCTION
The total volume of total hip and knee replacement surgery is on the up in the Australian Orthopaedic setting. According to the 2020 Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), a total of 51,163 total hip arthroplasty (THA) procedures and 66,729 total knee arthroplasty (TKA) procedures were performed in 2019. This represents an increment of 1.9% and 1.3% in total hip and knee arthroplasty surgery in 2019 respectively, compared to the previous year [1]. By 2030, the estimated demand for primary total knee replacements is projected to grow by 673% to 3.48 million with primary hip replacements by 174% to 572,000 in the USA [2]. Prosthetic joint infections (PJI), albeit infrequent, have been found to be accountable for 15% & 25% of failed hip and knee arthroplasties respectively [3,4]. These figures are actually increasing in terms of the percentage of revision arthroplasties carried out due to PJI’s compared to the previous figures in the AOANJRR 2020 report. The increasing numbers of peri-prosthetic joint infections is concerning. These infections come with a significant risk of complications ultimately leading to revision surgery with significant morbidity and mortality. Increased rates of failures for PJI are also associated with increased BMI in TKA surgery. In addition its physical and mental impact to the patient, there is a significant burden to our healthcare system. Thus, it is pivotal to accurately distinguish between an infected and non-infected prosthetic failure as the operative management differs significantly and requires appropriate surgical planning [5-7].
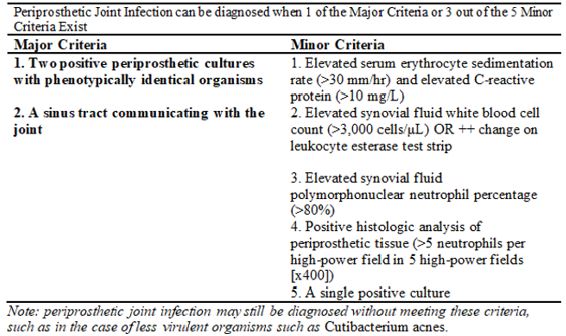
In our Orthopaedic department all revisions arthroplasty patients are subjected to a standardised diagnostic algorithm and cases reviewed weekly by the consultant team to ensure that an accurate working diagnosis is accompanied by a robust surgical plan. Patients who have a suspected PJI are also discussed and reviewed at our Infectious Diseases MDT clinic. These patients often present with non-specific symptoms. As a result, there are multiple different investigative tools (biochemistry, microbiological and radiological) which are utilised in formulating a working diagnosis of PJI. An ideal test would be a single test which is highly sensitive & specific, easy to perform and interpret, while also being time efficient [8]. Based on our MDT protocol, all cases have multiple intraoperative deep tissue biopsies taken with 5 specimens sent for prolonged bacterial culture (14 days) and 1 for histopathology. When the intraoperative diagnosis is in doubt frozen section (FS) tests are used to try and obtain a diagnosis [9-11]. However, this test has been found to be logistically challenging, time consuming and is often associated with false positive or negative results. In view of the importance in correctly identifying an infected periprosthetic joint, the Musculoskeletal Infection Society (MSIS) has incorporated both clinical data and six tests to create a definition of PJI. The MSIS Workgroup at their International Consensus Meeting defined the Standard diagnostic criteria for Peri-prosthetic Joint infection criteria is as summarised in Figure 1 below [12-14].
In our practice, we utilise the ICM criteria to identify patients with peri-prosthetic joint infections. The intraoperative factors which we observe in our practice include from surgical biopsies are:
A pathogen isolated on 2 or more separate peri-prosthetic tissue/fluid cultures obtained from the affected prosthetic joint OR Isolation of a pathogen in 1 peri-prosthetic tissue/ fluid culture & HP demonstrating >5 Neutrophils/ HPF in 5 HPFs on one of the deep tissue samples.
Studies have shown that the body’s innate immune system is triggered by the presence of pathogens that cause a peri-prosthetic joint infection leading to a cascade of protective pathways in the host [11]. Interestingly, white blood cells in synovial fluid can demonstrate a unique gene expression ‘signature’ that differentiates chronic infection from aseptic inflammation [15]. This innate immune system response has also been confirmed at the level of the proteome meaning that there could potentially be several different biomarkers that are more sensitive in diagnosing a peri-prosthetic joint infection than the tests that are currently available [5,16,17].
Synovasure test kit is one such commercially available test kit, which swiftly detects the presence of inflammatory protein AD-1 released by neutrophils in the synovial fluid aspirate. The protein is made by host neutrophils and therefore is not specific to any specific pathogen. Crucially, the test is unaffected by antibiotic therapy. Such characteristics make it an invaluable tool for intraoperative diagnosis of PJIs. Furthermore, a systematic review and meta-analysis by Wyatt in 2016 found that there is a high diagnostic accuracy when looking at chronic PJI’s for both AD-1 and Leucocyte Esterase tests. From this we know that AD-1 is extremely useful in diagnosing PJI’s, however, the existing evidence does not necessarily suggest that it is more or less valuable than current frozen section tests. Finally, the test is very quick and gives a result to the surgeon intra-operatively to assist in decision making during surgical procedures. The aim of this study is to ascertain how reliable Synovasure is in detecting chronic PJIs. This test was added to our existing tests routinely performed in diagnosing prosthetic joint infections.
METHODS
This is a retrospective pilot study that has been approved by our institutional ethics review board (SACHREC) with approval number 213.17 obtained. A sample size of 42 patients who were scheduled to undergo revision total hip or total knee replacements at our institution were included in this study from a time period of March 2017 to June 2018. All patients who were scheduled for revision surgery of both the hip or knee were included in this study. Only patients who were undergoing a 1st revision were included to exclude known 2 stage revisions for infection. In total there were 19 knee revision and 23 hip revision procedures planned. Symptoms that were reported by patients were primarily because of ongoing pain which amounted to 22 patients and symptoms of instability which amounted to 20 patients.
All patients that were enrolled in this study were treated using our standardised revision protocol and the guidelines of our Infection MDT clinic [18]. In addition to this, each patient was tested with the Synovasure kit intraoperatively. 1ml of synovial fluid was extracted with a sterile syringe and passed to a junior doctor or nurse in theatre who has been trained to use the testing procedure. Each patient’s synovial fluid was tested with the Synovasure test kit under strict sterile circumstances and results were recorded accordingly. The results were then compared with Lab results of deep tissue biopsies to compare and look for false positives and false negatives.
RESULTS
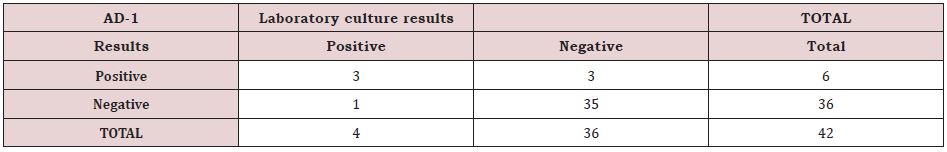
Overall, 6 patients out of 42 had a positive result on Synovasure biomarker testing 3 had positive laboratory cultures confirmed. Unsurprisingly pathogens varied between the 3 patients with one for each of Staphylococcus Epidermidis, Enterococcus Faecalis and Serovar Typhmurium. Three patients tested positive for Synovasure biomarkers but were negative on histology. Interestingly 2 of these cases had intra operative evidence of Metalosis.
There was only one false negative case with a positive laboratory culture for Staphylococcus Epidermidis. Therefore, from our results the Synovasure testing has a low level of sensitivity of 50% for positive results (3/6) cases. Sensitivity is however high with only 1 false negative out of 36 negative cases at 97%. Our Positive predictive value is 75% at 3 out of 4 cases and Negative Predicative Value is 89.7% at 35 correct predictions out of 39 predictive cases. These results are summarized in Table 1 below.
DISCUSSION
Complications following hip and knee arthroplasties are often divided into infective and non-infective causes. Thus, it is pivotal to correctly delineate an infected peri-prosthetic joint from one that is not in order to tailor subsequent management accordingly. The aim of our study was to test how reliable AD-1 is as a tool to correctly diagnose a PJI intra-operatively. We have also decided to utilize this test in assisting with intra-operative decision making when deciding between a single, two stage or repeat first stage revision arthroplasty for a suspected prosthetic joint infection. From our study, we have found that Synovasure correctly identified all the diseased patients who have periprosthetic joint infections with 100% sensitivity, with the limitation of a small sample size of 20 patients. It was also able to correctly identify 93.75% of patients who did not have periprosthetic joint infections potentially saving them from unnecessary antibiotic regimes and further operative intervention.
Deirmengian et al. [5] concluded that several synovial fluid biomarkers exhibited nearly ideal sensitivity, specificity and accuracy and could be a valuable tool for PJI diagnosis. A Metaanalysis by Taras et al. [19] concluded frozen section performs well in predicting a diagnosis of culture positive PJI but only had moderate accuracy in ruling out the diagnosis of PJI. Jacovides et al. [16] demonstrated promising results using synovial fluid molecular marker in diagnosis of PJI and also observed that frozen section requires specialized knowledge, can be logistically difficult and time inefficient to perform intra-operatively in patient under anaesthesia, as these patients naturally have multiple medical comorbidities. This is certainly similar to our experience within our own department. Bingham et al. [8] reported that AD1 was 100% sensitive and 95% specific in a study performed on 61 patients in detecting PJI. Frangiamore et al. [20] with a larger sample size of 102, concluded that AD1 test was 100% sensitive, 98% specific, with a PPV & NPV of 96 % &100% respectively. In a study conducted by Kasparek et al. [9] on 40 patients comparing AD1 & FS it was found that AD was 67% sensitive & 93% specific.
It is also worth commenting on the potentially limited effectiveness of the Synovasure test in patients who are obese. We know that such patient groups would be more likely to require a TKR due to the increased weight load passing through the joint during dynamic movement. Obesity has also been linked with a chronically lower baseline level of Alpha Defensins due to neutrophil dysfunction in these patients. As has been shown by Wilson et al. [21], these patients who are obese are the types of patients who are much more likely to suffer from a surgical site infection post-TKR. This, therefore, must also be taken into account when considering the effectiveness of biomarkers such as AD-1 in diagnosing PJI’s in patients with a BMI over 25 [22-25].
In our series we have shown that the Synovasure test is very useful at predicting negative cases and may allow surgeons to have the confidence to make intra-operative decisions with higher speed and certainty than using conventional frozen section analysis. However, the fact that false positives and negatives do exist confirms that this is a useful intraoperative test and not a substitute for the internationally recognized ICM diagnostic criteria for PJI. Interestingly Metalosis cases can have false positives adding to the diagnostic complexity our Infection MDT have experienced with these cases in the past [26-28].
CONCLUSION
We conclude that intra-operative AD1 testing is a user friendly, quick & reliable intra-operative tool to rule out suspicious chronic PJI. Pivotally, it is useful when contemplating intra-operatively between single or two stage revision arthroplasty and we aim to prove that it is reliable enough to replace frozen section as a replacement for intraoperative prosthetic joint infection diagnosis. Given the accuracy of AD1 tests as compared to final Laboratory culture results in our study we believe that it has a great potential as intra-operative decision-making tool for revision arthroplasty surgeons in the Australian setting.
REFERENCES
- (2020) Australian Orthopaedic Association National Joint Replacement Registry (AOAANJRR). Hip, Knee and Shoulder Arthroplasty.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89(4): 780-785.
- Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, et al. (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468(1): 45-51.
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, et al. (2009) The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am 91(1): 128-33.
- Deirmengian C, Hallab N, Tarabishy A, Della Valle C, Jacobs JJ, et al. (2010) Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res 468(8): 2017-2023.
- Ghanem E, Parvizi J, Burnett RS, Sharkey PF, Keshavarzi N, et al. (2008) Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am 90(8): 1637- 1643.
- Morgan PM, Sharkey P, Ghanem E, Parvizi J, Clohisy JC, et al. (2009) The value of intraoperative Gram stain in revision total knee arthroplasty. J Bone Joint Surg Am 91(9): 2124-2129.
- Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, et al. (2014) The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res 472(12): 4006-4009.
- Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, et al. (2016) Intraoperative diagnosis of periprosthetic joint infection using a novel alpha-defensin lateral flow assay. J Arthroplasty 31(12): 2871- 2874.
- Kwiecien G, George J, Klika AK, Zhang Y, Bauer TW, et al. (2017) Intraoperative frozen section histology: matched for musculoskeletal infection society criteria. J Arthroplasty 32(1): 223-227.
- Tzou P, De Gregorio E, Lemaitre B (2002) How Drosophila combats microbial infection: a model to study innate immunity and hostpathogen interactions. Curr Opin Microbiol 5(1): 102-110.
- Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, et al. (2011) New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469(11): 2992-2994.
- Parvizi J, Jacovides C, Zmistowski B, Jung KA (2011) Definition of periprosthetic joint infection: is there a consensus? Clin Orthop Relat Res 469(11): 3022-3030.
- Izakovicova P, Borens O, Trampuz A (2019) Periprosthetic joint infection: current concepts and outlook. EFORT Open Reviews 4(7): 482-494.
- Deirmengian C, Lonner JH, Booth RE (2005) The Mark Coventry Award: white blood cell gene expression: a new approach toward the study and diagnosis of infection. Clin Orthop Relat Res 440: 38-44.
- Jacovides CL, Parvizi J, Adeli B, Jung KA (2011) Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty 26(6 Suppl): 99-103 e1.
- Shahi A, Parvizi J (2016) The role of biomarkers in the diagnosis of periprosthetic joint infection. EFORT Open Rev 1(7): 275-278.
- Visvanathan A, Wilson CJ, Jackman E, Wong G and Krishnan J (2021) Design, Construction and Early Results of a Formal Local Revision Knee Arthroplasty Registry J Knee Surg 34(12): 1284-1295.
- Tsaras G, Maduka-Ezeh A, Inwards CY, Mabry T, Erwin PJ, et al. (2012) Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 94(18): 1700-1711.
- Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK (2016) Alpha-defensin accuracy to diagnose periprosthetic joint infection-best available test? J Arthroplasty 31(2): 456-460.
- Wilson CJ, Georgiou KR, Theodoulou A, Deakin AH, Krishnan J (2018) Surgical Site infection in overweight and obese arthroplasty Patients. J Orthop 15(2): 328-332.
- Balato G, Franceschini V, Ascione T, Lamberti A, D’Amato M, et al. (2017) High performance of alpha-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg Sports Traumatol Arthrosc. 26(6): 1717-1722.
- Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, et al. (2015) The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res 473(1): 198-203.
- Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, et al. (2015) The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res 473(7): 2229-2235.
- Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, et al. (2008) Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 23(7): 984-991.
- Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J (2012) Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27(8 Suppl): 61-5 e1.
- Li B, Chen F, Liu Y, Xu G (2017) Synovial fluid alpha-defensin as a biomarker for peri-prosthetic joint infection: A systematic review and meta-analysis. Surg Infect (Larchmt) 18(6): 702-710.
- Pupaibool J, Fulnecky EJ, Swords RL, Sistrunk WW, Haddow AD (2016) Alpha-defensin-novel synovial fluid biomarker for the diagnosis of periprosthetic joint infection. Int Orthop 40(12): 2447-2452.
Article Type
Research Article
Publication history
Received Date: March 17, 2022
Published: April 27, 2022
Address for correspondence
Christopher Wilson, Department of Orthopaedic Surgery, Consultant Orthopaedic Surgeon, Director of Arthroplasty Research, Flinders Medical Centre, Australia
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Conor S, Ashwath S, Vikas S, Christopher W. Reliability of Alpha-Defensin 1 Assay in Detecting Periprosthetic Joint Infections: A Pilot Study. 2022- 4(2) OAJBS.ID.000442.