Potential Bio-Control Agent Serratia sp. SCP Isolated from Rhizosphere Soil, Mahbubnagar, Telangana
ABSTRACT
The use of plant growth promoting microbes as bio-control agents and bio-fertilizers gives eco-friendly and inexpensive alternatives to the use of chemicals in agriculture. Plant growth promoting rhizobacteria (PGPR) promotes the plant growth by various mechanisms such as production of plant growth hormones, phosphate solubilization, nitrogen fixation, suppression of phytopathogens by production of antifungal metabolites and by many other ways. A PGPR with a potential as a biological agent, phosphate solubilizer, producing growth promoting hormones, compatibility with fungicides and herbicides and salt tolerance is desirable. A free-living bacterial strain bacteria was isolated from the rhizosphere of Ricinus communis L. It was classified by morphological and biochemical tests, and further by 16S rDNA sequencing. It had shown close similarity with Serratia marcescense. Serratia sp. SCP displayed a broad spectrum of antifungal activity in vitro against phytopathogens such as Fusarium oxysporum f. sp., Ricinus communis, Alternaria solani and Rhizoctonia solani by production of antimicrobial compounds. Many mechanisms which involved in biocontrol of Serratia were identified. Strain SCP produces Indole Acetic acid (IAA), ammonia and siderophores for iron competition. In addition, it is an effective phosphate solubilizer and has compatibility with broad spectrum of fungicides and antibiotics. It promoted the plant growth in association with arbuscular mycorrhiza.
KEYWORDS
PGPR; Serratia sp.; SCP; Ricinus communis; AM Fungi
ABBREVATIONS
IAA: Indole Acetic acid; PGPR: Plant growth promoting rhizobacteria; PGR: Plant Growth Regulators; PSM: Phosphate Solubilizing Microorganisms; ISR: Induced Systemic Resistance; P: Phosphorus; PSM: Phosphate Solubilizing, Microorganisms; NA: Nutrient Agar; SE: Solubilizing Efficiency
INTRODUCTION
Many microorganisms reside in the rhizosphere of the plants. Plant growth promoting rhizobacteria (PGPR) can actively colonize plant roots and increase plant growth [1]. The growth promotion mechanisms may be direct i.e., production of growth hormones, phosphate solubilization, nitrogen fixation or indirect, such as suppression of deleterious organisms by siderophore production or secretion of antifungal metabolites [2]. The mechanisms with which PGPR influence plant growth may vary from species to species and strain to strain. The performance is well demonstrated in the laboratory and green house conditions, but results are inconsistent in the field conditions.
Biocontrol of different insect genera including Anomala, Costelytra and Phyllophaga by Serratia entomophila is also known [3]. Chitinase, from S. marcescens, is in use to control plant diseases [4]. Biofertilizer mainly act in three different ways, either fixing atmospheric nitrogen, or provide plant growth regulators (PGR), while others solubilize and translocate water insoluble soil nutrient including macro (P&K), and micro (Zn & Cu) elements. Soil pH either alkaline or acidic, decreases phosphorus availability to plants. Phosphate solubilizing microorganisms (PSM) solubilize insoluble phosphate to inorganic phosphorus to act natural buffering system [5]. Serratia plymuthica is most frequently associated with plants. This organism has been isolated from the rhizosphere of grass [6], wheat [7], maize [8], oilseed rape [9], grape [10], melon [11], onion [12], Brassica sp. [13], tomato [14] and endophyte from the endorhiza of potato [15] and on the phyllo sphere of spring wheat [16]. Research over the past years has demonstrated that induced systemic resistance (ISR) can be a potential mechanism by which PGPR demonstrated biological disease control [17]. ISR is dependent on colonization of the root system by sufficient number of PGPRs. Previous studies have shown that proteamaculans 1-102 promotes soybean-bradyrhizobia inoculation and growth, but the mechanism is unknown [18].
Biological control is a potential way of reducing chemical use in agriculture. Biocontrol bacteria can mediate their role in disease suppression through various mechanisms including competition for nutrients and niches [19], production of antimicrobial metabolites and induced systematic resistance in the host plants. Some biocontrol PGPR protects the plants by activating gene encoding defense enzymes-peroxidase, Chitinase, phenylalanine, ammonia-lyase, β-1, 3-glucanase and others involved in synthesis of phytoalexin [19]. In addition to biological nitrogen fixation, phosphate solubilization is equally important. Phosphorus (P) is major essential macronutrients for biological growth and development. Microorganisms offer a biological rescue system capable of solubilizing the insoluble inorganic P of soil and make it available to the plants. The ability of some microorganisms to convert insoluble phosphorus (P) to an accessible form, like orthophosphate, is an important trait in a PGPB for increasing plant yields. The rhizosphere phosphate utilizing bacteria could be a promising source for plant growth promoting agent in agriculture. Among the heterogeneous and naturally abundant microbes inhabiting the rhizosphere, the Phosphate solubilizing, microorganisms (PSM) including bacteria have provided an alternative biotechnological solution in sustainable agriculture to meet the phosphorous demands of plants. PSM include largely bacteria and fungi. The most efficient PSM belong to genera Bacillus, Rhizobium and Pseudomonas amongst bacteria, and Aspergillus and Penicillium amongst fungi.
Nearly 40% of world’s surface has salinity problems. Most of the saline areas confined to the tropics and the Mediterranean region and has made the salt tolerance an urgent priority for the future of agriculture. The productivity of crops is greatly affected by salt stress. Highly alkaline (pH greater than 8.0) soils tending to be high in sodium chloride, bicarbonate and borate, are often associated with high salinity, reduces nitrogen fixation. Saline conditions reduce the ability of plants to absorb water, induce many metabolic changes causing rapid reduction in growth rate, similar to those caused by water stress. If salt-tolerance cannot be improved, by perforce vast amounts of soils may be left uncultivated. The failure of nitrogen fixing activity of some nitrogen-fixing organisms in high salinity clearly inhibits the induction of lupines. In such soils, microorganisms tolerating high concentration of salt and yet capable of fixing nitrogen are of importance in increasing its fertility. The halotolerant microorganisms are effective in the treatment of waste from tannery industry or pickle industry [20]. These organisms are isolated from sources such as marine environment, soils, rhizosphere or industrial waste. They are also known to be the potential sources of extracellular enzymes with novel properties, useful for diverse industrial applications. Hence the objective of the present study is isolation and characterization of high salt tolerant, non-halophilic microorganisms from food samples and evaluation of their characteristics under stress conditions.
Arbuscular mycorrhizal fungi constitute an important component of the soil microbial community and are extremely successful fungi that form mutualistic symbioses with about two thirds of all plant species. They improve plant nutrition and promote plant diversity, help to control pests and fungal pathogen and affect the fitness of plants in polluted environments.
MATERIAL AND METHODS
Test Organisms
The soil used for bacterial isolation was collected from rhizosphere soil of Castor plant near Mahabubnagar. The processed soil sample was serially diluted, spread plated on full strength nutrient agar and incubated at 28 ºC for 48 h. A total of 59 different colonies were isolated on nutrient agar (NA) and were purified with repeated culturing and maintained in 20% glycerol at -80 ºC. A potential isolate was screened and selected on the basis of halo zone produced in Pikovskaya agar, Salt tolerance at 6%, IAA production and antifungal activity. Strain was assessed for phenotypic and molecular 16S rDNA characterization.
Bacterial Identification and Characterization
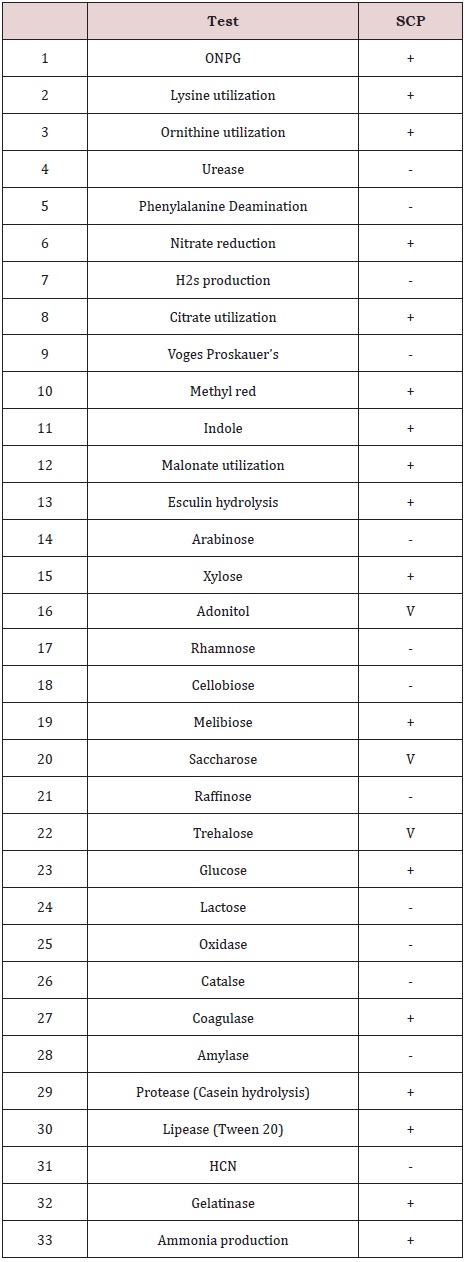
Isolated strain was subsequently differentiated by gram reaction, microscopic observation, biochemical tests (ONPG, Lysine utilization, Ornithine utilization, Urease, Phenylalanine, Deamination, Nitrate reduction, H2S production, Citrate utilization, Voges Proskauer’s, Methyl red, Indole, Malonate utilization, Esculin hydrolysis, Arabinose, Xylose, Adonitol, Rhamnose, Cellobiose, Melibiose, Saccharose, Raffinose, Trehalose, Glucose, Lactose, Oxidase, Catalase, Coagulase, Amylase, Protease, Casein hydrolysis), Lipase (Tween 20), HCN, Gelatinase, (using Hi-25 Kit, Himedia, Mumbai) and 16S rDNA sequence.
16s rDNA Sequencing
16S rDNA gene was amplified using SCP genomic DNA as a template by PCR using the primers pair FD1 (5′-AGA-GTT-TGATCC- TGG-CTC-AG-3′) and RP2 (5′-ACG-GCT-ACC-TTG-TTA-CGACTT- 3′) designed in the conserved region. Purified genomic DNA was isolated from organism and used as template in PCR reaction (DNA template 100 ng; primer 5 pm; Big Dye terminator dye 2 μl of genomic DNA in total reaction volume of 20μl, with final magnesium concentration of 1.5 mM). The amplification conditions were as follows 33 cycles of 94 ºC, denaturation 30 sec; annealing 53 ºC, 30 sec; extension 60 ºC, 4 min incubation at 72 ºC. The 1499 bp PCR product was sequenced. Sequencing was performed with 5 different primers viz., 16SEQ2R, INS16SREV, 16SEQ3F, 16SEQ4F and 16SEQ4R designed in the conserved regions on 16S rDNA. The 16S rDNA similarity sequences searches were performed using BLASTN in the GenBank database of the NCBI website.
Antagonistic Ability
The invitro antagonistic assay was performed using dual culture method on YMA medium. Agar discs (5 mm) of Rhizoctonia solani, Fusarium oxysporum f. sp. Ricinus communis and Alternaria solani isolated from 7-day old culture was disposed at the center of Petri dishes and the bacterial strain was streaked in a square form around the agar disc at 4 cm distance. The antagonistic activity of the studied bacterial strain was estimated by the inhibition of the fungal growth monitored by measuring the diameter in centimeter of the colony until 10 days at 28 ºC in BOD incubator. The experiment was carried out in triplicate.
Quantitative Estimation of IAA Production
Luria-Bertani Broth medium (Hi-Media) was used containing L-tryptophan (1 g/litre). The pH was adjusted to 7.5 with 1 N NaOH before autoclaving. To determine the amount of IAA produced a colorimetric technique was performed using the Van Urk Salkowski reagent (2% 0.5 M Fecl3 in 35% Perchloric acid). Isolate was grown in no-agar LBT medium and incubated at a 30 ºC temperature for 72 h in a rotary shaker (90 rpm). Later, the culture broth was centrifuged at 5000 rpm for 10 min. The supernatant was mixed with salkowaski reagent (2:1). Development of pink color indicates IAA production and optical density was recorded at 530 nm spectroscopy after 30 min.
Using LB medium, different concentrations of IAA (0, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 ppm) were prepared using standard IAA (Hi-Media). Further, these concentrations were treated with salkowaski reagent as above for measuring developed.
Phosphate Solubilization
The isolate was screened for phosphate solubilization as per the methodology described by [21] on modified Pikovskaya agar (Glucose-10 g, Ca3(PO4)2-5 g, KCl-0.2 g, MgSO4-0.1 g, MnSO4-trace, FeSO4- trace, Yeast extract 0.5 g, Agar-15 g, Distilled water- 1 L, pH- 7.0) and the plates were incubated at 30±1 ºC for 48-96 h. Phosphate solubilization is indicated by the formation of a solubilization or a clear zone around the bacterial colony. A loop full of SCP culture was placed on the center of agar plates and incubated at 30±1 ºC for 7 days. The appearance of transparent halo zone around the bacterial colony indicated the phosphate solubilizing activity of the bacterium. Halo surrounding the colonies were measured and the solubilizing efficiency (SE) was calculated by the following formula: SE= (Solubilization diameter (S)/Growth diameter (G)) x 100.
NH3 Production
Bacterial isolate was tested for the production of ammonia in peptone water. Overnight growth culture of bacteria was inoculated in 10 ml peptone broth and incubated at 30 ± 0.1 ºC for 48 hr in incubator shaker. After incubation 0.5 ml of Nessler’s reagent was added. The change of faint yellow to dark brown color indicated the production of ammonia [22].
Siderophore production
Siderophore production was determined by the chrome azurol S (CAS) assay [23]. Overnight cultures in LB medium at 28 ºC were seeded onto CAS agar plates and incubated for 2 days at 28 ºC after which the diameter of the orange haloes, indicating production of Siderophore was measured.
Hydrogen cyanide
Bacterial isolate was screened for the production of hydrogen cyanide by adapting the method of [24]. Briefly, nutrient broth was amended with 4.4 g glycine/L and bacteria were streaked on modified agar plate. A Whatman filter paper no.1 soaked in 2% sodium carbonate in 0.5% picric acid solution was placed in the top of the plate. Plates were sealed with parafilm and incubated at 28 ± 2 ºC for 4 days. Development of orange to red color indicates HCN production.
Antibiotic susceptibility test by Disc diffusion method
Disc diffusion method was used for antimicrobial assay using antibiotics. Sterile twenty milliliters of molten Mueller Hinton agar were poured into the Petri plate (Hi-Media). After solidification, 100 μl of the inoculum (1x108 cfu /ml) was inoculated uniformly over agar plate. The different antibiotic discs (Hi-Media, Dodeca G-V Plus, Dodeca Universal-IX, Hexa G-Plus 3) were saturated with 100 μl of the upper layer of the seeded agar plate. The plates were incubated overnight at 32±1 ºC. Microbial growth was determined by measuring the diameter of zone of inhibition. The result was obtained by measuring the zone diameter. The experiment was carried out thrice and the mean values are presented.
Antifungal susceptibility test by agar well diffusion Method
Agar well diffusion assay is the key process used to evaluate the antifungal potential of fungicides. In the present study six systemic fungicides i.e., carbendazim, tebuconazole+trifloxystrobin, hexaconazole, propiconazole, azoxystrobin and benomyl were used. Petri dishes (100 mm) containing 20 ml of Mueller Hinton agar (MHA) were seeded with approximately 100 μl inoculums of bacterial strains (inoculums size was adjusted so as to deliver a final inoculum of approximately 108 CFU/ml). After solidification of the media, wells of 6 mm diameter were cut into media using a sterilized cork borer. 100 μl of each fungicide was poured into respective well and the plates were incubated at 32 ºC overnight. The experiment was performed thrice under strict aseptic conditions to insure consistency of all findings. Fungicidal activity on inoculums was expressed in terms of the mean of diameter of zone of inhibition (in mm) produced at the end of the incubation period.
Sodium Chloride (NaCl) Tolerance
The bacterial isolates were inoculated separately on specific agar medium containing 1%, 2%, 3%, 4%, 4.5, 5%, 6%, 7%, 8%, 9%, 10% M concentrations (NaCl). Four replications of the plates for each isolate were maintained along with control. After 48 hrs of incubation, observations for survival and growth of inoculum were started [25]. Promising isolates were repeated for their confirmations.
Inoculum Preparation and Application
Bacterium: To prepare the inoculums, initially Serratia sp. SCP was cultured in Nutrient Broth medium (Himedia, M002- 100G; ingredients: peptic digest of animal tissue - 4.00 g/l; sodium chloride - 5.00 g/l; beef extract - 1.50 g/l; yeast extract - 1.50 g/l, final pH 25 ºC -7.4±0.2) and was allowed to grow properly, with shaking at 37 ºC at 120 rpm for 48 h. At the end of the log phase, bacterial culture was centrifuged at 10,000 rpm for 15 min and the supernatant was discarded, selecting the bacterial pellet. Pellet was scraped into sterile distilled water. The aqueous suspensions were diluted as necessary to maintain the bacterial concentration at 108 CFU/ml. The aqueous suspension was then applied as a soil drench, at 100 ml/plant to the rhizosphere of castor seedling both in field and potted conditions one month after transplantation. Application was performed at an interval of one month and three applications were carried out. The bacterial suspension was applied once more as a soil drench prior to pruning.
Fungus: Culture of Fusarium oxysporum was grown in sandmaize meal medium (Maize meal: sand: water (1:9:5 w:w:v) in autoclavable plastic bags (sterilized at 20 Lb pressure for 20 min) for a period of three weeks at 28 0C until the mycelia completely covered the substrate.
Preparation of bio-formulation of Serratia sp. SCP
The bio-formulation was prepared using rice husk. For the preparation of bio-formulation, 2.5 g of carboxy methyl cellulose sodium salt (Himedia) was added to 250 g of rice husk, pH was adjusted to 7 by adding calcium carbonate and sterilized twice for 30 min. 100 ml of aqueous bacterial suspension (at a concentration of 1 x 1010 CFU/ml) was added to the mixture, was dried under shade to reduce the moisture to less than 20%. Formulation was packed in polythene bags, sealed and stored at room temperature. Survivability of Serratia sp. SCP was checked at a regular interval of one month for a period of nine months using direct plating method in nutrient agar medium.
Green House Experiment
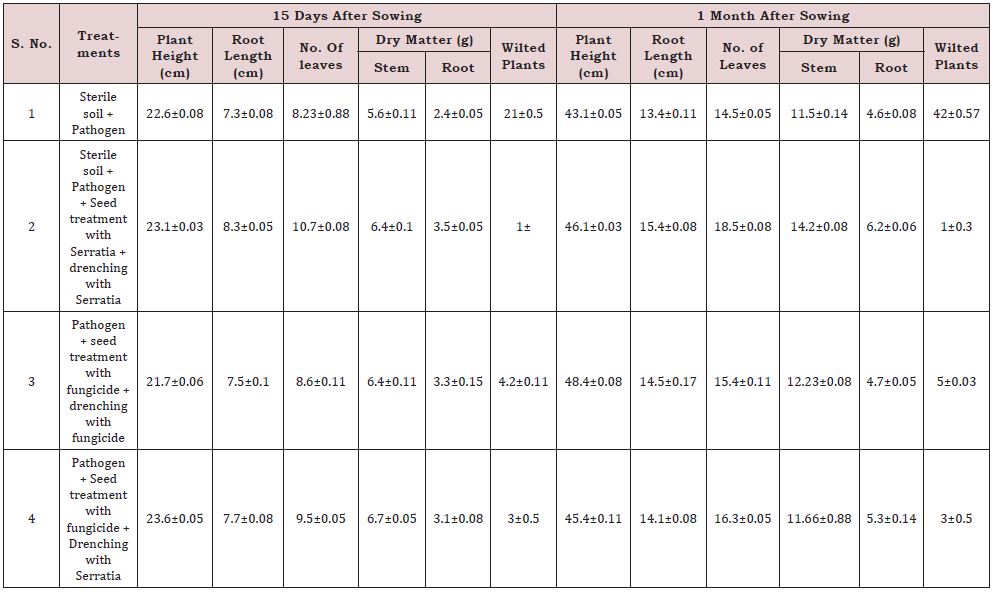
The experiment consisted of four treatments i.e., control (sterile soil + pathogen), sterile soil + pathogen + seed treatment with Serratia + Serratia inoculums added to soil + drenching with Serratia, pathogen + seed treatment with fungicide + drenching with fungicide, and pathogen + seed treatment with fungicide + drenching with Serratia in a pot size of 60 x 60 x 40 cm3. Data of plant height, root length, no. of leaves, dry matter produced (stem & root) and no. of wilt plants at 155 and 30 days of sowing. Three replicates (total of 100 plants) were maintained for each treatment.
RESULTS
Bacterial Identification
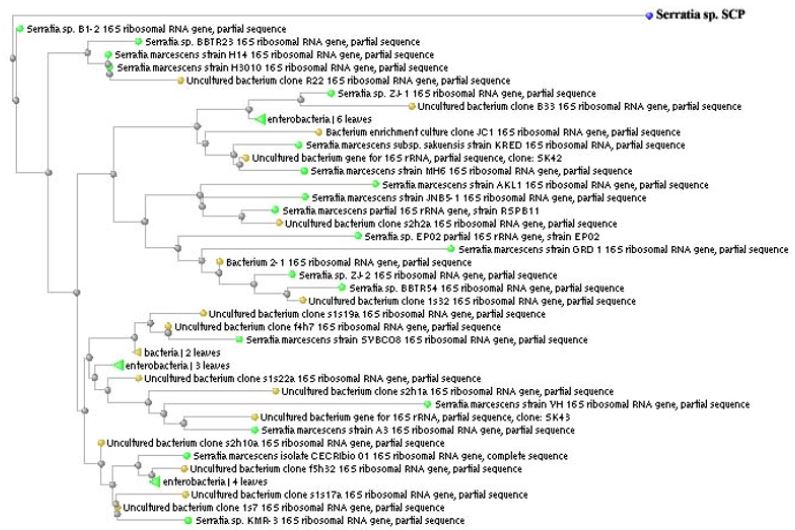
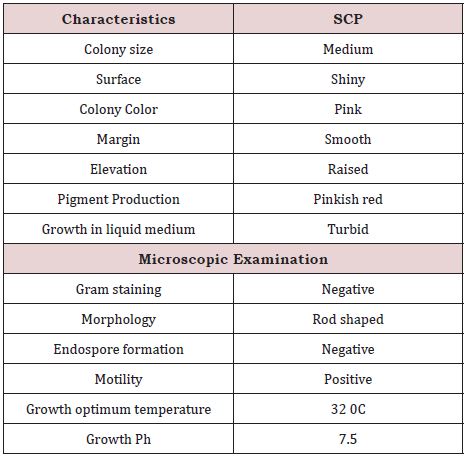
The bacterial strain was gram negative, motile rods, with circular smooth margin, raised and pinkish red in colour (Table 1). The strain was positive for ONPG, lysine utilization, ornithine utilization, nitrate reduction, citrate utilization, methyl red, indole, malonate utilization, esculin hydrolysis, xylose, melibiose, glucose, coagulase, protease (casein hydrolysis), lipase (Tween 20), gelatinase and ammonia production, and negative for Urease, phenylalanine, deamination, H2S production, voges proskauer’s, arabinose, rhamnose, cellobiose, raffinose, lactose, oxidase, catalase, amylase and HCN, and intermediate (11-89% positive) for adonitol, saccharose and trehalose. It was able to grow over a wide range of temperatures 15-40 ºC, with optimum at 32 ± 0.5 ºC. It had a pH tolerance over the range of 4-10, with optimum 7.0 ± 0.5 (Table: 2). Further, 1,499 bp of 16S rDNA was cloned and sequenced. The sequence of 16S rDNA of strain SCP was deposited in GenBank under accession number JX276738. The 16S rRNA similarity sequence searches revealed that strain SCP shares the highest similarity to members of the Serratia marscens in GeneBank database (Figure 1).
Antifungal Activity
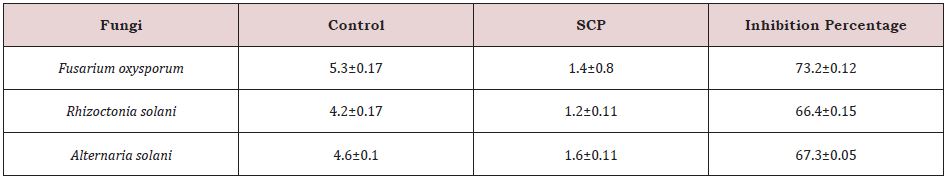
Dual culture bioassays demonstrated that three phytopathogenic fungal (Fusarium oxysporum, Rhizoctino solani and Alternaria) isolates tested were suppressed with strain SCP on PDA plates, indicated by different sizes of inhibition. The per cent inhibition was more for Fusarium oxysporum (73.2) followed by Alternaria solani (67.3) and Rhizoctino solani (66.6), (Figure 2); (Table 3).
IAA Production
The strain in culture medium containing L-Tryptophan source, produced growth hormone IAA to an extent of 49 ppm as detected by salkowski reagent under colorimetry as compared to the synthetic standard IAA (10-100 ppm); (Figure 3).
Phosphate Solubilization
On Pikovaskaya’s agar, the strain showed very distinct, clear, and transparent P solubilization zone (16 mm) and recorded high Solubilization efficiency (SE) of 200% Table 4.
Ammonia Production
Ammonia production is an important trait of PGPR that indirectly influence the plant growth. The strain was producing ammonia.
Siderophore Production
The strain was producing siderophores as indicated by the orange halo on CAS-agar plate.
Sensitivity to Fungicides
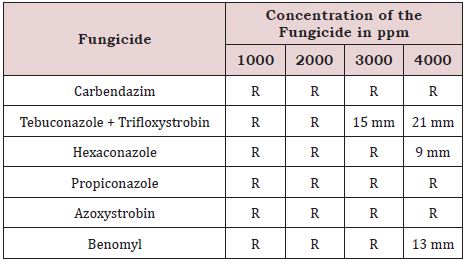
The test fungicides carbendazim, propiconazole and azoxystrobin could not inhibit the growth of Serratia sp. SCP even at 4000 ppm under in vitro conditions. Tebuconazole + trifloxystrobin (3000 ppm), hexaconazole (3000 ppm) and benomyl (3000 ppm) inhibited the bacterial growth (Table 5).
NaCl Tolerance
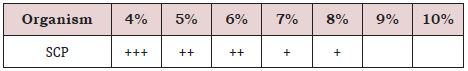
Of the different concentrations of NaCl (0-10% w/v), the strain could tolerate up to 8% (Table 6).
Antibiotic Sensitivity Test
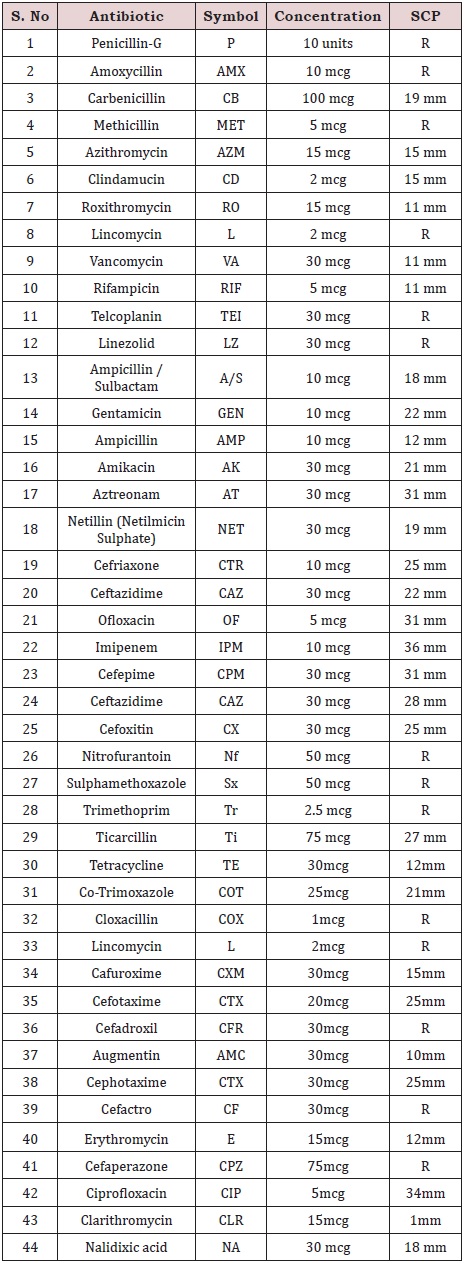
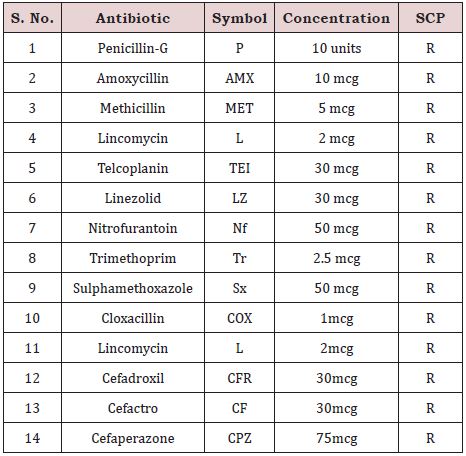
Out of 44 antibiotics used, Serratia was resistant to 14 antibiotics (Figure 4); (Table 7) (penicillin-G, amoxycillin, methicillin, lincomycin, telcoplanin, linezolid, nitrofurantoin, sulphamethoxazole, trimethoprim, cloxacillin, lincomycin, cefadroxil, Cefactro and cefaperazone) and susceptible to 30 antibiotics(Carbenicillin, Azithromycin, Clindamucin, Roxithromycin, Vancomycin, Rifampicin, Ampicillin /Sulbactam, Gentamicin, Ampicillin, Amikacin, Aztreonam, Netillin (Netilmicin Sulphate), Cefriaxone, Ceftazidime, Ofloxacin, Imipenem, Cefepime, Ceftazidime, Cefoxitin, Ticarcillin, Tetracycline, Co-Trimoxazole, Cafuroxime, Cefotaxime, Augmentin, Cephotaxime, Erythromycin, Ciprofloxacin, Clarithromycin and Naidixic acid); (Table: 8).
The results of the experiment indicated the treatment sterile soil + pathogen + seed treatment with Serratia + drenching with Serratia, was found to be most effective over other in terms of Fusarium wilt control and plant growth after those 4 methods sterile soil+ pathogen + seed treatment with fungicide + Drenching with Serratia show also better plant growth (Table 9).
DISCUSSION
Isolation and Molecular Identification
In the present study, the strain SCP was characterized morphologically and biochemically and later subjected to 16S rDNA sequencing. This strain identified as Serratia sp. SCP (Gene Bank accession no.) whose rDNA sequence was similar to genus Serratia marcescense stored in the NCBI database.
Biological Control
Most studies on the biocontrol of plant pathogens focused on the use of antagonistic rhizobacterial strains belonging to the genus Pseudomonas or Bacillus. However, several Serratia strains have been demonstrated to be effective bio-control agents against a range of plant diseases [26]. S. marcescense NBR 11213 was reported to induce plant growth promotion and biological control of foot and root rot of betelvine caused by Phytophthora nicotiaeneae [27,28] also reported that three strains of PGPRs-Serratia sp. J12, fluorescent Pseudomonas J3 and BB11-provided disease control in tomato against Tomato wilt and increased yield. Numerous studies have shown a substantial increase in plant growth and seed yield following inoculation with PGPR strains [29]. S. marcescens not only promoted plan growth but also reduced intensity of brown root rot disease [30]. In the present study Serratia Sp. SCP reduced the three phytopathogenic fungal mycelia i.e., Fusarium oxysporum, Rhizoctonia solani and Alternaria solani. S. marcescens was shown to have the ability to secrete IAA, produce sideriohore, solubilize phosphate and was also antagonistic to fungal pathogen. It was also a non-HCN and chitinase producing strain [31]. Phosphate solubilizing ability and antagonistic activity of S. marcscens against root rot pathogens was confirmed [30].
Siderophores synthesized by microbial communities of soil supply iron to plants that possess the mechanisms for its uptake under iron deficient conditions [32]. The phytohormone, the IAA synthesized from the transamination and decarboxylation of tryptophan, primarily in the young leaves and seeds, controls cell division, root initiation, phototropism, geotropism and apical dominance in plants [33]. Bacterial IAA has the potential to interfere with any of these processes by input of the IAA into the plant’s auxin pool.
Strain SCP can produce an array of secondary metabolites such as chitinase and protease that inhibit the growth of plant pathogens and synthesize siderophores to competitively acquire ferric iron. It is also able to produce the plant growth hormone indole-3-acetic acid. These data implied that besides direct antagonism against plant pathogens, such as antibiosis, lysis and competition; the interactions with host plants such as production of plant auxin IAA might be also involved in biocontrol activity indirectly, which is similar to well-studied plant rhizospheric bacteria S. plymuthica HRO-C48, IC1270 and IC14 [34,35,26].
Phosphate Solubilization
The ability of PGPRs to convert insoluble phosphorus (P) to an accessible form is an important trait for increasing the plant yields. The fact that certain microbes are capable of dissolving relatively insoluble phosphatic compounds has opened the possibility of inducing microbial solubilization of the phospahates in the soil [36,37]. Rhizobacteria solubilize the mineral P in the rhizosphere and hence, provided soluble P to the plants. The cause of the mineral P solubilization could probably be due to secretion of organic acids such as gluconic, 2-ketogluconic, oxalic, citric, acetic, malic, and succinic, etc. [38]. The strain SCP is a potential phosphate solubilizer. The ammonia released by the rhizobacterial strain plays a signaling role in the interaction between PGPR and plants and also increases the glutamine synthetase activity [39].
Tolerance to Fungicides
In our study, the Serratia sp. SCP exhibited an unusual tolerance to higher concentrations of the six systemic fungicides carbendazim, tebuconazole + trifloxystrobin, hexaconazole, propiconazole, azoxystrobin and benomyl. Microorganisms that developed resistance to xenobiotics, such as pesticides, are frequently capable of biodegrading them and are able to bioremediate the soils [40]. In agriculture usage of fungicides was increased enormously. PGPR showing tolerance/resistance is highly to fungicides is highly desirable.
Salt Tolerance
Soil salinity in arid regions is frequently an important limiting factor for cultivating agricultural crops. Serratia marcescense sp. SCP expressed the salt tolerance up to 8%, indicating they are new novel strains qualified by adaptation to environment and thereby acquiring additional traits. Earlier salt tolerant PGPR Bacillus strains were reported such as Bacillus clausii [41], different sp. of Bacillus [42,43], Bacillus cereus [20] and Lactococcus lactis (subsp. lacti).
Antibiotic Resistance
Antibiotic assay revealed that Serratia marcescense was susceptible to all the antibiotics used except for Penicillin-G, Amoxycillin, Methicillin, Lincomycin, Telcoplanin, Linezolid, Nitrofurantoin, Sulphamethoxazole, Trimethoprim, Cloxacillin, Lincomycin, Cefadroxil, Cefactro and Cefaperazone.
Green House Experiment
The green house experiment revealed that seed treatment with Serratia, addition of Serratia inoculum soil and drenching with Serratia is found to be most effective in controlling Fusarium wilt over other treatments. The results also indicate that Serratia is acting as a growth promoter, it is evident from plant height, root length and dry matter production. In agriculture use of bio-control agents will enhance the yield levels by promoting the growth and protect the environment by avoiding use of chemical fertilizers and others.
CONCLUSION
Plant growth promoting rhizobacteria (PGPR) promotes the plant growth by various mechanisms such as production of plant growth hormones, phosphate solubilization, nitrogen fixation, suppression of phytopathogens. Serratia sp. SCP displayed a broad spectrum of antifungal activity in vitro against phytopathogens such as Fusarium oxysporum, Alternaria solani, Helminthosporium and Rhizoctonia solani by production of antimicrobial compounds. Many mechanisms which involved in biocontrol of Serratia were identified. Strain SCP produces Indole Acetic acid (IAA), ammonia and siderophores for iron competition. In addition, it is an effective phosphate solubilizer and has compatibility with broad spectrum of fungicides and antibiotics. It promoted the plant growth in association with Arbuscular Mycorrhiza. Therefore, SCP is desirable as a potential biological agent, phosphate solubilizer, producing growth promoting hormones, compatibility with fungicides.
ACKNOWLEDGMENT
All the authors are thankful to Prof. L.B. Laxmikanth Rathod Vice-chancellor of the Palamuru University, Telangana for their encouragement and support.
REFERENCES
- Kloepper JW, Schroth MN (1978) Plant growth promoting bacteria on radishes. International Conference on Plant Pathogenic bacteria. Angers, France 2: 879-882.
- Kloepper JW, Zehnder GW, Tuzun S, Murphy JF, Wei G, et al. (1996) Toward agricultural implementation of PGPR mediated induced systematic resistance against crop pests. In: Wenhua T, Cook R J, Rovira A (eds). Advances in biological control of plant diseases. Beijing: China Agricultural University Press. P 165-174.
- Nunez Valdez ME, Calderon MA, Aranda E, Hernnanadez L, Ramirez- Gama RM, et al. (2008) Identification of a putative Mexican strain of Serratia entomophila pathogenic against root damaging larvae of Scarabaeidae (Coleoptera). Appl Environ Microbol 74: 802-810.
- Ordentilich A, Elad Y, Chet I (1998) The role of chitinase Serratia marcescens in biocontrol of Sclerotium rolfii. Phytopathology 78: 84-88.
- Narula N, Kumar V, Behl RK, Deubel A, Gransee A, Merbach W (2000) Effective of Phosphate solubilizing Azotobacter chroococcum on N, P, K uptake in P-responsive wheat genotypes grown under greenhouse conditions. J. Plant Nutr Soil 163: 393-398.
- Alstrom S, Gerhardson B (1987) Characteristics of a Seeatia plymuthica isolate from plant rhizosperes. Plant and Soil 103: 185-189.
- Astrom S, Gerhardson B (1988) Differential reactions of wheat and pea genotypes to root inoculation with growth-affecting rhizosphere bacteria. Plant and Soil 109: 263-269.
- Lucon CMM, Melo IS (2000) Effect of seed bacterization on the development of maize plants and Fusarium moniliforme control. Fitopatologia Brasileira 25(3): 529-537.
- Kalbe C, Marten P, Berg G (1996) Strains of the genus Serratia as beneficial rhizobacteria of oil see rape with antifungal properties. Microbiological Research 151(4): 433-439.
- Chemin L, Ismailov Z. Haran S, Chel I (1995) Chitinolytic bacteria Enterobacter agglomeans antagonistic to fungal plant pathogens. Applied and environmental Microbiology 61(5): 1720-1726.
- Kamensky M, Ovadis M, Chet I, Chernin L (2003) Soil-Born strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia Sclerotiorum diseases. Soil Biology and Biochemistry 35(2): 323-331.
- Park C, Shen S (2002) Biocontrol of phytophthora blight of pepper employing Serratia plymuthica A21-4 and effect of soil population of phytophthora capsici on the root colonization of the antagonistic bacteria. Bullentin OILB/SROP 25(10): 327-330.
- Carlot M, Giacomini A, Casella S (2002) Aspects of plant-microbe interactions in heavy meatal polluted soil. Acta Biotechnologica 22(1- 2): 13-20.
- Formmel MI, Pazos GS, Nowak J (1991) Plant-growth stimulation and biocontrol of Fusarium wilt (Fusarium oxysporum f.sp. lycopersici) by coinoculation of tomato seeds with Serratia plymuthica and Pseudomonas sp. Fitopatologia 26(2): 66-73.
- Berg G, Krechel A, Duitz M, Sikora RA, Ulrich A, et al. (2005) Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungal. FEMS Microbiology Ecology 51(2): 215-229.
- Legard DE, Quliken MP Mc, Whipps JM, Fenlon JS, Fermor TR, et al. (1994) Studies of seasonal changes in the microbial populations on the phyllopshere of spring wheat as a prelude to the release of a genetically modified microorganism. Agriculture, Ecosystems & Environment 50(2): 87-101.
- Bai Y, Souleimanow A, Smith DL (2002) An inducible activator produced by a Serratia proteamaculans Strain and its soybean growth promoting activity under greenhouse condition. J Exp Bot. 53(373): 1495-1502.
- M’Piga P, Belanger RR, Paulitz TC, Benhamou N (1997) Increased resistance to Fusarium Oxysporum f.sp. radicis-lycopersici in tomato plants related with the endophytic bacterium Pseudomonas fluorescens strain 63-28. Physiol Mol Pl Pathol 50(5): 301-320.
- Kubo M, Hiroe J, Murakami M, Fukami H, Tachiki (2001) Treatment of hypersaline-containing wastewater with salt-tolerant microorganisms. Journal of Biosciences and Bioengineering 91(2): 222-224.
- Gupta RS, Rekha S, Aparna S, Kuhad RC, Saxena RK, et al. (1994) A modified plate assay for screening phosphate solubilizing microorganisms. Journal of General and Applied Microbiology 40(3): 255-260.
- Cappuccino JG, Sherman N (1992) Biochemical activities of microorganisms. In: Microbiology, A Laboratory Manual. The Benjamin / Cummings Publishing Co. California, USA.
- Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1): 47-56.
- Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiologia Plantarum 1(2): 142-146.
- Benson HJ (1990) Temperature: Lethal effects. In Microbiology Applications. Wm. C., Brown Pub. USA., pp: 125-126.
- Vleeschauwer D. de, Ho¨fte M (2007) Using Serratia plymuthica to control fungal pathogens of plant. CAB Rev 2: 046.
- Lavania M, Chauhan PS, Chauhan SV, Singh HB, Nautiyal CS (2006) Identification of plant defense enzymes and Phenolics by treatment with plant growth promoting rhizobacerial Serratia marcescens NBRII213. Curr Microbiol 52: 363-368.
- Guo JH, Qi HY, Guo YH, Ge HL, Gong LY, et al. (2004) Biocontrol of tomato wilt by plant growth promoting rhizobacteria. Bio Cont 29(1): 66-72.
- Wani PA, Khan MS, Zaidi A (2007) Synergistic effects of the inoculation with nitrogen fixing and phosphate solubilizing bacteria on the performance of field grown chickpea. J Plant Nutr Soil Sci 170(2): 283- 287.
- Chakraborty U, Chakraborty BN, Tongden C, Roychowdhury P, Basnet M (2004) Assessment of tea rhizopshere microorganisms as plant growth promoters. Tea 25: 38-47.
- Chakraborty U, Chakraborty B N, Chakraborty AP (2010) Influnce of Serratia marcescens TRS-1 on growth promotion and induction of resistance in Camelia sinesis against Fomes lamaoensis. J Plant Interactions 5(4): 261-272.
- Indiragandhi P, Anandham R, Madhaiyan M, Tongmin S (2008) Characterization of plant growth promoting traits of bacteria isolated fromlarval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Current Microbiology 56(40): 327-333.
- Khan MS, Zaidi A, Wani PA, Ahemad M, Oves M (2009) Functional diversity among plant growth promoting rhizobaceria. In: Khan MS, Zaidi A, Musarrat J, Microbial strategies for Crop Improvement, Springer 105-132.
- Kamensky M, Ovadis M, Chet I, Chernin L (2003) Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biol Biochem 35(2): 323-331.
- Ovadis M, Liu X, Gavriel S, Ismailov Z, Chet I, et al. (2004) The global regulator genes from biocontrol strain Serratia plymuthica IC1270: cloning, sequencing, and functional studies. J Bacteriol 186(15): 4986- 4993.
- Nahas E, Banzatto DA, Assis LC (1990) Fluorapatite solubilization by Aspergillus niger in vinasse medium. Soil Biol Biochem 22(8): 1097- 1101.
- Bojinova D, Velkova R, Grancharov I, Zhelev S (1997) The bioconversion of Tunisian phosphorite using Aspergillus niger. Nutr Cyc Agroecosyst. 47(3): 227-232.
- Zaidi A, Khan MS, Ahemad M, Oves M, (2009) Plant growth promotion by phosphate soilubilizing bacteria. Acta microbiologica et immunologica hungarica 56(3): 263-284.
- Chitra RS, Sumitra VC, Yash DS (2002) Effect of different nitrogen sources and plant growth regulators on glutamine synthetase and glutamate synthase activities of radish cotyledons. Bulgarian Journal of Plant Physiology 28(3-4): 46-56.
- Kumar S, Mukerji KG, Lal R (1996) Molecular aspects of pesticide degradation by microorganisms. Critical Review in Microbiology 22(1): 1-26.
- Asha Devi NK, Balakrishnan K, Gopal R, Padmavathy S (2008) Bacillus clausii MB9 from the east coast regions of India: Isolation, biochemical characterization and antimicrobial activity. Current Science 95: 627-636.
- Arahal DR, Carmen Marquez M, Volcani BE, Schleifer KH, Ventosa A (1999) Bacillus marismortui sp. nov., a new moderately halophilic species from the Dead Sea. International Journal of Systematic Bacteriology 49(2): 521-530.
- Palmisano MM, Nakamura LK, Duncan KE, Istock CA, Cohan F (2001) Bacillus sonorensis sp. nov., a close relative of Bacillus licheniformis, isolated from soil in the Sonoran Desert, Arizona. International Journal of Systematic and Evolutionary Microbiology 51(5): 1671-1679.
- Rasa S, Jornsgard B, Abou-Taleb H, Christiansen (2001) Tolerance of Bradyrhizobium sp. (Lupini) strains to salinity, pH, CaCO3 and antibiotics. Letters in Applied Microbiology 32(6): 379-383.
Article Type
Research Article
Publication history
Received Date: May 10, 2022
Published: July 08, 2022
Address for correspondence
Prof. Dr. Pavan Kumar Pindi, Department of Microbiology, Palamuru University, India
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Shiva Shanker A, Madhusudan DR, Padmavati K, Pavan KP. Potential Bio- Control Agent Serratia sp. SCP Isolated from Rhizosphere Soil, Mahbubnagar, Telangana. 2022- 4(4) OAJBS.ID.000465.
Figure 1: Phylogenetic tree for Serratia sp. SCP.
Figure 2: Dual culture assay of Alternaia and Fusarium oxysporum f.sp. ricini at 7 days.
Figure 3: Quantitative estimation of IAAA production by Serratia sp. SCP.
Figure 4: Antibiotic sensitivity test.
Table 1: Phenotypic characteristics of Serratia sp. SCP.
Table 2: Biochemical tests.
Table 3: Antifungal activity.
Table 4: Phosphate solubilizing ability of SCP.
Table 5: Effect of fungicides on bacterial growth (biocontrol agent).
Table 6: NaCl tolerance test from 4% to 7 %.
Table 7: Antibiotic Sensitivity test.
Table 8: Serratia sp SCP showing resistance below the antibiotics.
Table 9: Greenhouse experiment.