Pharmacology Associated with the Appearance of Systemic Lupus Erythematosus: Review Article
ABSTRACT
Drug-induced lupus erythematosus (DILE) is a syndrome triggered by an uncontrolled immune response that occurs after exposure to certain medications. It represents approximately 10-15% of all cases of patients diagnosed with idiopathic systemic lupus erythematosus (SLE), highlighting that the pathogenic mechanisms that lead to autoimmunity are still partially described. The clinical manifestations are usually associated with general signs or symptoms such as weight loss, arthralgia, myalgia, nondeforming arthritis, temperature rises and serositis, and may even cause skin alterations and, rarely, cardiac, renal or central nervous system involvement. The diagnosis must be carried out carefully given the similarity that it could present with SLE and in this document you will find a systematic review of general data related to the problem in question, which will allow expanding the associated knowledge and promote the timely identification of all cases.
KEYWORDS
DILE, Drugs, Immune response, Mechanisms, Similarity, SLE
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease of multifactorial origin that is closely associated with various genetic or environmental factors, in addition to various clinical manifestations such as skin rashes, neuropsychiatric disorders, pulmonary serositis, arthritis and glomerulonephritis. [1]. Its appearance begins predominantly in youth and early adulthood, presenting peaks of maximum incidence between 15 and 55 years, which is estimated at approximately 1 to 23 per 100,000 inhabitants per year. The female/male ratio is 9:1 respectively [2] and its management is based on the implementation of combined pharmacological therapies that systematically include the administration of corticosteroids [3]. Over the years, it has been possible to identify that various types of drugs have the ability to generate an excessive immune response in patients with various types of genetic predisposition for SLE, thus unmasking pathologies that were asymptomatic prior to the use of the drug, exacerbating the of the condition already diagnosed or finally, triggering syndromes that present similar manifestations [1]. This has been collectively referred to as drug-induced lupus or DILE, which was first described in 1945 in association with sulfadiazine administration. This situation develops in patients with no history of autoimmune disease and represents 10 to 15% of all reported cases [1,2].
DILE is considered a rare and reversible process characterized by the presence of signs and symptoms similar to those found in patients diagnosed with SLE; however, the epidemiology as well as the clinical course differ significantly. More than 90 drugs related to this condition have been reported, with hydralazine and procainamide being the most commonly mentioned [3,4]. Currently there are no unanimously established classification criteria for diagnosis due to the diversity of manifestations that can be found, however, under medical suspicion, the presence of criteria such as being treated with a suspected drug should be documented. at least one month duration; present symptoms such as arthralgia, fever, weight loss, serositis and rash and finally, determine the positivity of ANA and anti-histones in the absence of other specific markers [4,5]. It is important to mention that, despite the fact that the establishment of the causal relationship represents a challenge for healthcare personnel, the clinical improvement after drug withdrawal, as well as the negativization of autoantibodies, is essential for the comprehensive approach.
METHODOLOGY
A systematic search of original articles, case reports and bibliographical reviews is carried out in databases specialized in the exposed topic such as ScienceDirect, Pubmed, Elsevier, Scielo and Medline. Search keywords such as: “LUPUS”, “DILE”, “induction”, “medications” and “autoimmune” are used, carefully selecting a total of 16 bibliographical references in the Spanish and English languages that were relevant for the development of the study. this review article.
RESULTS
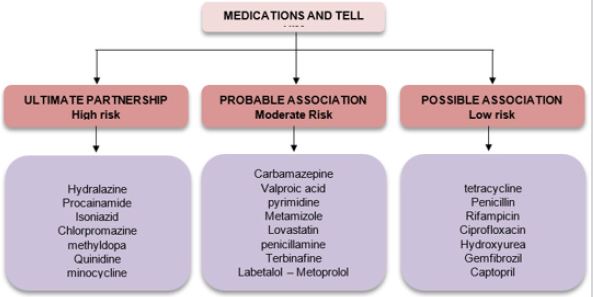
Currently, approximately 90 drugs related to the induction of DILE have been described for which two broad classifications known as the level of risk and the association with the disease have been determined. In the first section, subgroups of high, moderate, and low risk (<5%, 1-5%, 0.1-1% respectively) will be found, while in the second, definitive, probable and possible association will be discussed [2]; (Figure 1). It is important to mention that there is no uniformity in the pharmacology, characteristics or indication of medications related to the presentation of this entity, since they belong to at least ten different categories, among which antiarrhythmics, antihypertensives, antipsychotics, antibiotics, anticonvulsants stand out antithyroid, anti-inflammatory, statins and anti-TNF-a. Only five of all related drugs have the ability to regularly generate antinuclear antibodies, with procainamide being the most frequently associated drug [90% positive ANCAS, 30% DILE] followed by hydralazine [4] (Figure 1).
Regarding the pathophysiological mechanisms of the disease, there is no single recognized cause of the induction of autoimmunity. However, it is important to mention that there is a certain degree of related genetic susceptibility, since it has been established that relatives of patients diagnosed with SLE are more likely to generate the drug-induced form. It has also been reported that slow acetylators managed with hydralazine or procainamide generate ANA positivity more quickly and progress more frequently to symptomatic DILE [2]. Currently, there are 4 hypotheses related to this process:
a. Hapten hypothesis: Drugs and their metabolites could have
the ability to generate haptens by binding to proteins, which
would trigger an excessive immune response by T and B cells [4].
Additionally, it has been established that this union could produce
an antigenic transformation in proteins [3].
b. Direct cytotoxicity hypothesis: Through in vitro studies,
with little evidence, the hypothesis has been postulated that the
metabolites involved in the process could generate direct alteration
in apoptotic cells through the deterioration of their degradation
and clearance capacity [3]. This would generate loss of tolerance
towards self-antigens and direct cell death.
c. Lymphocyte activation hypothesis: spleen cells from
murine specimens exposed in vitro[4]at high concentrations
of procainamide and hydralazine have shown an increased
proliferative response towards autologous antigen-presenting
cells, which facilitates the differentiation of B cells into antibodysecreting
plasma cells [2].
d. Alteration of central immune tolerance: Through in vitro
studies, it has been shown that when drugs related to the induction
of lupus are applied in the thymus, an alteration is generated at
the level of tolerance of autoantigens. This is derived from the sustained creation of antichromatin antibodies that cause selfantigens
previously presented by the major histocompatibility
complex to thymocytes not to be recognized peripherally by T
lymphocytes [1,3]. The clinical spectrum of DILE varies from
circumscribed cutaneous signs to systemic involvement, which is
generally reported as mild. Its onset is insidious and can debut from
the first days of exposure to the drug until years after the start of
pharmacological treatment. There are three forms of presentation
for the pathology which are known as: systemic DILE, subacute
cutaneous DILE and chronic cutaneous DILE [4,6].
TELL Systemic
It is mainly characterized by the presence of general symptoms and signs such as temperature rises, myalgias, arthritis in small joints that does not generate deformity and serositis [5]. Mucocutaneous manifestations are usually common, often showing classic signs such as malar rash, discoid lesions, hair loss or the presence of oral ulcers [5,6]. This clinical pattern is typical of cases induced by hydralazine and the severity is directly related to the time of exposure to the drug. Severe complications such as pericarditis, cardiac tamponade, pulmonary infiltrates, renal involvement, and neurological involvement are likely but rare [7].
Subacute Cutaneous DILE
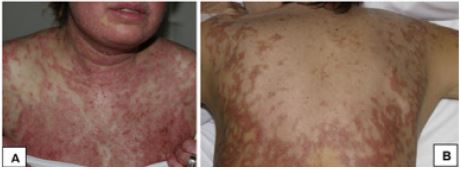
The clinical and serological presentation is similar to that evidenced in SLE and, additionally, it also agrees that the presentation is more prevalent in older women [8]. The skin findings are characterized by scaly eruptions with a papulosquamous pattern located in areas frequently exposed to the sun, such as the face, chest, and arms; however, the lesions can spread to affect nonphotoexposed areas, such as the lower third of the back (Figure 2). Extracutaneous manifestations are usually absent, and most patients have complete remission of symptoms approximately 8 weeks after stopping the offending drug. There may be positivity in anti-Ro/SSA and anti-La-SSB, which will become negative at approximately 8 months [1].
Chronic Cutaneous DIL
This clinical presentation is usually manifested by the presence of classic discoid-type lesions in areas that are frequently exposed to the sun, such as the face, chest, and arms. Systemic manifestations are usually absent, and resolution occurs in the first 5 months after drug suspension [8,9].
DISCUSSION
Drug-induced systemic lupus erythematosus was first reported in 1945 in a patient receiving sulfadiazine therapy. Later, in 1952, a series of cases related to hydralazine was reported. It is estimated that approximately 15,000 to 30,000 new cases occur each year in the United States, representing around 10-15% of the number of patients with SLE [10]. Whereas its incidence is variable depending largely on the medication associated with the pathology. The proportion of the appearance of the condition does not change significantly between men and women, however, it has been possible to show a higher prevalence in white patients, as well as in older adults [11]. DILE is considered a medical disorder whose clinical presentation depends on the person’s intrinsic conditions, as well as various pharmacological associations. However, there are a number of risk factors related to its appearance, such as drug metabolism and characteristics. their immunogenicity. Similarly, regardless of the mechanisms, the clinical features will always be mediated by autoimmunity.
On the other hand, according to Aguirre [4] hydralazine, procainamide, isoniazid, methyldopa, quinidine, minocycline and chlorpromazine are the only ones associated by means of controlled prospective studies. The manifestations and the serological profile are associated with the type of drug, so it is not possible to clearly describe a single usual pattern. In systemic involvement, the presence of general symptoms such as weight loss, temperature rises, arthralgia, myalgia and serositis while, in the skin condition, alterations related to erythema nodosum, purpura, urticaria, necrotizing vasculitis, discoid lupus and, less frequently, malar rash will be described, photosensitivity, oral ulcers or alopecia [12]. Rubin [11] mentions that necrotizing vasculitis is more frequent when the drug administered belongs to pharmacological families such as angiotensin-converting enzyme inhibitors, calcium channel blockers and anti-tumor necrosis factor; additionally, Aguirre and López [4] assure that renal, pulmonary and central nervous system involvement is rarely described. The diagnosis of DILE usually represents a challenge for healthcare personnel, greatly hindering the timely and rapid perception of the cases that have occurred, which is why, in order to facilitate the process, various authors, including Valenzuela [5]. They tell us that, in general, the clinical condition can be suspected in patients with no history of autoimmune conditions, with positive antinuclear antibodies (present in more than 80% of cases) [4] and with symptoms indicative of SLE. In the same way, they assure that a complete medical study must be carried out that contains a blood count, metabolic panel, urinalysis and serological profile [5] (antinuclear antibodies, anti-double-stranded DNA, antihistone, anti-Sm and anti-RO, anti-La). In this part, it is important to mention that different medications have the capacity to generate an autoimmune response with induction of autoantibodies, but their positivity has not been associated, in all cases, with the presence of the disease [13].
On the other hand, Wales [10] ensure that hematological abnormalities and hypocomplementemia evidenced in SLE are rare in DILE. In most of the reported case series, the clinical picture resolves satisfactorily after discontinuation of the drug, however, the recovery time will largely depend on the type of drug, the extent of the manifestations, and the characteristics of the patient [8]. The reality is that there are no randomized trials that have been responsible for evaluating the approach to therapeutic management in patients with DILE; however, most authors agree with the idea of the benefit that the use of effective medications for idiopathic SLE could represent in these patients [12]. In general, arthralgia and arthritis could be controlled with non-steroidal antiinflammatory drugs (NSAIDs), cutaneous, musculoskeletal, and constitutional symptoms with hydroxychloroquine (if they do not resolve spontaneously after 8 weeks) and in some cases, especially in patients with pleurisy or severe pericarditis, use of systemic glucocorticoids may be beneficial [13]. Antinuclear antibodies could remain detectable for up to 12 months [14].
Differential diagnoses for DILE are usually first SLE followed by hypersensitivity reactions. As previously clarified, SLE and DILE differ not only in the extent of target organ damage but also in the characteristics of the clinical manifestations and autoimmunity. For their part, Pretel [10] comment that hypersensitivity reactions can be easily ruled out since there is no evidence of the production of specific T lymphocytes directed towards the drug or antibodies, clinical manifestations are evident for months or even years after the initial exposure to the drug, the clinical picture will depend on the accumulated dose and, finally, in case the drug was administered again in the patient [15-20].
CONCLUSION
Drug-induced lupus erythematosus is considered an autoimmune disorder resulting from exposure to specific medications. The manifestations may be associated with systemic or cutaneous involvement and resolve, in most cases, within a few days of having withdrawn the associated compound. At this point, it is important to highlight that various drugs have the ability to induce the production of autoantibodies without triggering the disease as such, so it must be recognized that the positivity of these elements does not always require the suspension of the drug. Finally, with the continuous development of medical therapies, it is expected that in the coming years the number of reported cases will increase significantly, so it is important that the healthcare personnel of the health services be trained for the effective recognition of the various forms of presentation, as well as in the establishment of comprehensive management.
REFERENCES
- Revus J, Valeyrie AL (2016) Drug Reactions. Dermatology: main diagnoses and treatment. Elsevier pp. 149-171.
- Limper A (2022) Drug-Induced pulmonary disease. Murray and nadel’s textbook of respiratory medicine. Elsevier pp. 1378-1394.e16.
- Pretel M, Marqués L, España A (2014) Drug-induced lupus erythematosus. Actas Dermosyphiliogr 105(1): 18-30.
- Zamorano MAA, Pedrera RL, Lozano MJC (2010) Drug-induced lupus. Medicine Clinic 135: 124-129.
- Valenzuela R, García P, León P, Hernández P, Pereira C (2012) Autoimmunity induced by drugs: about a case. Rev Chile Reumatol 28(4) :200-204.
- Marzano AV, Lazzari R, Polloni I, Crosti C, Fabbri P, et al. (2011) Druginduced subacute cutaneous lupus erythematosus: Evidence for differences from its idiopathic counterpart. Br J Dermatolo 165(2): 335- 341.
- Borchers AT, Keen CL, Gershwin ME (2007) Drug-induced lupus. Ann N Y Acad Sci 1108: 166-182.
- Antonov D, Kazandjieva J, Etugov D, Gospodinov D, Tsankov N (2004) Drug-induced lupus erythematosus. Clin Dermatol 22(2): 157-166.
- Vasoo S (2006) Drug-induced lupus: an update. Lupus 15(11): 757-761.
- Batchelor J, Welsh K, Mansilla R, Dollery T, Hughes GR, et al. (1980) Hydralazine-induced systemic lupus erythematosus: influence of hla-dr and sex on susceptibility. The Lancet 1(8178): 1107-1109.
- Rubin RL (2015) Drug-induced lupus. Expert Opinion on Drug Safety 14(3): 361-378.
- Marzano AV, Vezzoli P, Crosti C (2009) Drug-induced lupus: An update on its dermatologic aspects. Lupus 18(11): 935-940.
- Pérez DLM, Retamozo S, Flores CA, Kostov B, Roberto PA, et al. (2017) Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry). Expert Opinion on Drug Safety 16(11): 1255- 1271.
- Vaglio A, Grayson PC, Fenaroli P, Gianfreda D, Valeria B, et al. (2018) Drug-induced lupus: traditional and new concepts. Autoimmunity Reviews 17(9): 912-918.
- Galindo M, Molina RA, Alvarez JLP (2017) Systemic Lupus Erythematosus (I). Etiopathogenesis. Clinical manifestations. Natural history. Diagnostic tests. Differential diagnosis. Medicine (Spain) 12(25): 1429-1439.
- Ondarza Vidaurreta RN (2017) Systemic lupus erythematosus (SLE). Journal of Biochemical Education (REB) 36.
- Laurinaviciene R, Sandholdt LH, Bygum A (2017) Drug-induced cutaneous lupus erythematosus: 88 new cases. Eur J Dermatol 27(1): 28-33.
- Zeitjian V, Mehdizadeh A (2017) ANA-negative hydralazine-induced pericardial effusion. Case Rep Med 2017: 3521541.
- Sarkar R, Paul R, Pandey R, Roy D, Sau TJ, et al. (2017) Drug-induced lupus presenting with myocarditis. J Assoc Physicians India 65(6): 110.
- Kelly AS, Harpe GP, D’Arcy C, Lally A (2018) Drug-induced lupus erythematosus secondary to pirfenidone. Br J Dermatol 178(6): 1437- 1438.
Article Type
Mini Review
Publication history
Received Date: August 25, 2022
Published: October 11, 2022
Address for correspondence
Lorena Florez Arroyo, General Physician, Universidad del Magdalena, Colombia
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Lorena FA, Leonardo FAB, Jose LDD, Andrés FPV, Alejandro OZ, et al. Pharmacology Associated with the Appearance of Systemic Lupus Erythematosus: Review Article. 2022- 4(5) OAJBS.ID.000496.
Figure 1: Classification according to association and risk of medications related to drug-induced lupus.
Figure 2: Skin involvement in drug-induced lupus erythematosus. Cases secondary to the use of anticonvulsants. A: Papulosquamous psoriariform lesions on the chest and face. B: Squamous and erythematous annular lesions on the back [3].