Exosomal miRNA Research Technology for Combating Ischemic Stroke
ABSTRACT
Neurological dysfunction caused by ischemic stroke has brought a heavy economic burden to society and families. There is still a lack of specific treatment methods for ischemic stroke in clinical treatment. Exosomes have good compatibility and are easy to cross the blood-brain barrier. At the same time, exosomes can deliver contents to recipient cells and affect the biological function of recipient cells, which may provide new ideas for the treatment of ischemic stroke. As a part of exosome contents, miRNAs have attracted extensive attention. This article reviews the research progress of exosome miRNA in the treatment of ischemic stroke and the potential of targeted delivery of exosome miRNA in the treatment of ischemic stroke to provide a reference for the future research direction and the selection of therapeutic targets for haemorrhagic stroke.
KEYWORDS
Exosomes; Mirna; Ischemic stroke
INTRODUCTION
Stroke is the leading cause of mortality and disability worldwide and the second leading cause of death after ischemic heart disease [1]. Stroke can be divided into ischemic stroke and haemorrhagic stroke. Ischemic stroke is the most common type, accounting for about 62% of the total number of strokes worldwide and more than 80% of the total number of ischemic strokes in China [1,2]. Due to the limitation of the treatment time window, most patients fail to receive adequate treatment in the acute phase of ischemic stroke, resulting in severe neurological deficits. There is still a lack of adequate clinical treatment for patients with ischemic stroke beyond the treatment window, so it is urgent to seek new treatment methods. The blood-brain barrier makes safe and effective drug delivery a significant difficulty in treating ischemic stroke. Exosomes have attracted extensive attention in preclinical studies due to their unique characteristics. MicroRNA (miRNA), as one of the exosome’s genetic materials, has become a potential therapeutic target for treating ischemic stroke.
TREATMENT STATUS OF ISCHEMIC STROKE
Ischemic stroke refers to the reduction or interruption of blood supply to the brain due to various reasons, leading to ischemia and hypoxia of brain tissue, resulting in neuronal death, activation of microglia and astrocytes and a series of changes [3]. The pathology of ischemic stroke includes the formation of the ischemic core and penumbra. The key to stroke treatment is rescuing the ischemic penumbra neurons. Currently, the most effective treatment for ischemic stroke is intravascular reperfusion. Methods to achieve reperfusion after stroke include recombinant tissue plasminogen activator, intravenous thrombolytic therapy with RT-PA, and mechanical endovascular thrombectomy [4]. However, RTPA has a strict time window (≤4.5h) limitation, thrombolysis contraindications, and the risk of bleeding transformation. However, endovascular mechanical thrombectomy applies only to patients with large artery occlusion and some patients with thrombolysis contraindications [5,6]. Therefore, it is necessary to find a new specific treatment for patients outside the acute treatment window to promote the recovery of neurological function. Exosomes have attracted more attention in treating ischemic stroke due to their characteristics such as carrying genetic material, low immunity against phytophthora, good histocompatibility, high delivery efficiency and easy passage through the blood-brain barrier [7].
OVERVIEW OF EXOSOMES AND mRNA
Exosomes are single-layer membrane vesicles with diameters ranging from 30 to 200 nm secreted by living cells, containing proteins, lipids, nucleic acids, glycoconjugates, etc. Exosomes are a kind of extracellular vesicles [8]. Exosomes have stable bimolecular phospholipid structures, which can protect the biological activities of their contents from destruction. Exosomes play an essential role in immune response, tumor progression and neurodegenerative diseases by delivering their contents to neighboring or distant receptor cells and regulating the function of receptor cells [9]. At the same time, exosomes are expected to provide new treatment ideas for ischemic stroke due to their excellent compatibility and easy passage through the blood-brain barrier [10].
MiRNA is an endogenously produced single-stranded noncoding RNA molecule with a length of 21-23 nucleotides [11], which can regulate gene expression at the post-transcriptional level by inhibiting the expression of messenger RNA (mRNA) or promoting mRNA down-regulation [12]. MiRNA plays an important role in cell growth, proliferation, differentiation, apoptosis and other biological processes. In recent years, with the deepening of research, miRNAs in exosomes have attracted more and more attention. In animal models, miRNAs can be packaged into exosomes or microcapsules and secreted into the extracellular microenvironment for longdistance intercellular communication [13]. Many studies have shown that miRNAs in exosomes play an important role in diagnosing, treating and treating diseases [14-16].
Exosomes are highly heterogeneous in size, contents, function and cell origin. Changes in cell microenvironment and intrinsic biology may affect the content of exosomes and their contents [7]. Exosome packaging miRNA is not a random process and has an active sorting mechanism [15]. Studies have shown that exosomes’ miRNA expression profiles differ from those of parental cells, and highly enriched exosomal miRNAs may play an essential role in disease progression and treatment [9]. The expression level of miRNA in exosomes also changes under different physiological conditions. These characteristics of exosomes and the miRNAs they carry lay the foundation for the miRNAs in exosomes to become potential therapeutic targets for ischemic stroke.
miRNAS IN EXOSOMES
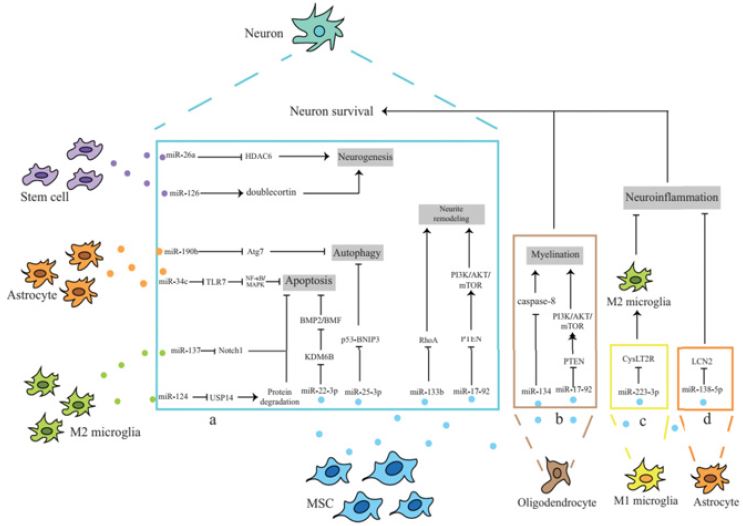
Treatment of ischemic stroke Exosome-loaded miRNAs have become a hot topic for treating central nervous system diseases. Exosomes can transport miRNAs with biological functions, and because of their inherent biological characteristics, exosomes have become a new therapeutic tool. After ischemic stroke, exosome miRNAs exert neuroprotective effects in different ways (including inhibiting inflammatory response, apoptosis, autophagy, and promoting neurite remodelling); (Figure 1).
Inhibition Of Inflammatory Response
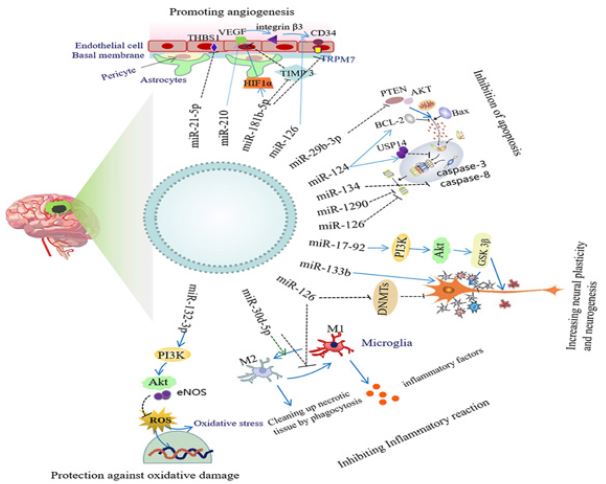
Neuroinflammation plays an important role in the occurrence and development of ischemic stroke. The inflammatory response has two aspects: on the one hand, activated inflammatory cells can engulf dead cells or debris and promote tissue repair; On the other hand, the overactivated inflammatory response will further aggravate the damage of neurons [17]. After the haemorrhagic stroke, Toll-like receptor 4(TLR4) is activated on inflammatory cells, leading to neuroinflammation and secondary nerve damage. Mesenchymal stem cells (Mesenchymal stem cells), Mir-542-3p carried by exosomes of Mesenchymal stem cells can reduce the glial inflammatory response and neuronal apoptosis by inhibiting TLR4[18]. In addition, Mir-221-3p carried by exosomes of bone marrow mesenchymal stem cells (BMSC) can reduce activated transcription factor 3 (ATF3), Reduce inflammation and neuronal injury in brain tissue after ischemic stroke in middle cerebral artery occlusion (MCAO) mice [19].
After ischemic stroke, microglia, the innate immune cells of the central nervous system, can differentiate into the “classically activated” pro-inflammatory M1 phenotype and the “vicarious activated” anti-inflammatory M2 phenotype, thus playing a role in nerve injury or protection [20]. Scientist found that MSC exosomes loaded with Mir-223-3p could induce microglia to transform from M1 phenotype to M2 phenotype by inhibiting the expression of Cysteinyl leukotriene receptor 2(CysLT2R) [21]. It then suppresses the inflammatory response. In addition, Mir-30D-5p carried in exosomes of Adipose-derived stem cells (ADSC) can promote the transformation of microglia into M2 phenotype by inhibiting autophagy and reducing inflammatory response after ischemic nerve injury [22]. After oxygen and glucose deprivation (OGD), ADSC-derived exosomes overexpressing Mir-126 were applied to BV2 microglia. Exosomes were found to inhibit the activation of BV2 cells and the expression of inflammatory factors. Promote the recovery of neurological function after haemorrhagic stroke [23]. In addition, Scientist found that Mir-146A-5p in human umbilical cord mesenchymal stem cells and exosomes produced by mesenchymal stem cells can reduce microglia-mediated neuroinflammation, reduce infarct size and improve neurobehavioral defects after ischemic stroke by inhibiting IRAK1/TRAF6 signaling pathway [24].
After ischemic stroke, astrocytes are activated as reactive astrocytes. Although the anti-toxic effect of reactive astrocytes limits the expansion of lesions and releases nutrients, the inflammatory response of reactive astrocytes to ischemic stroke can aggravate ischemic lesions, and the formation of glial scars also hinders the remodeling of neurites [25]. Mir-138-5p carried by BMSC-derived exosomes inhibits astrocyte inflammation and attenuates ischemic brain injury by down-regulating Lipocalin 2(LCN2) [26]. In addition, Scientist found that Mir-181c-3p in cortical neuron exosomes down-regulated the expression of C-X-C motif ligand 1 (CXCL1) through in vivo and in vitro experiments [27,28]. It can inhibit the inflammatory response of astrocytes and has a protective effect on ischemic brain injury as shown in Figure 2.
Inhibition Of Apoptosis
Apoptosis is the main cause of neuronal death in ischemic penumbra after stroke. Scientist demonstrated through in vivo and in vitro experiments that Mir-22-3p in ADMSC-derived exosomes could inhibit the BMP2/BMF axis by inhibiting the expression of KDM6B, thereby reducing neuronal apoptosis after ischemic stroke [29]. Scientist found that compared with exosomes derived from human neural stem cells, interferon-γ (IFN-γ) -induced exosomes derived from human neural stem cells are functionally superior and can significantly reduce neural cell apoptosis and promote cell proliferation and survival [30]. It plays a protective role after ischemic stroke, which may be related to exosomal miRNAs (such as Mir-206, Mir-133A-3p and Mir-3656). researcher found that in exosomes derived from M2 microglia, Mir-124 enhanced proteasome activity by inhibiting the expression of downstream target ubiquitin-specific protease 14(USP14) [31]. Increased degradation of misfolded proteins can significantly reduce neuronal apoptosis and promote survival after stroke. M2 microglia can also inhibit the expression of Notch1 through Mir-137 in its exosomes, thereby reducing neuronal apoptosis, improving neurological deficits, and alleviating cerebral ischemia-reperfusion injury [32]. In addition, Mir-34c in astrocytes-derived exosomes can downregulate NF-κB/MAPK signaling pathway by inhibiting TLR7, thereby inhibiting the apoptosis of N2a cells (neuroblastoma cell line), promoting the proliferation of N2a cells, and alleviating cerebral ischemia-reperfusion injury [33].
Inhibition of Autophagy
The lack of oxygen and sugar supply caused by cerebral ischemia can lead to the activation of the autophagy process, and an appropriate autophagy response can degrade or recycle misfolded proteins and damaged organelles. In contrast, an over-activated autophagy response will further aggravate cell damage [34]. Studies have shown that astrocyte-derived exosomes can improve neuronal injury after ischemic stroke by inhibiting autophagy, thus playing a neuroprotective role [35]. Scientist found that the expression of Mir-190b in astrocyte-derived exosomes was increased, and it could inhibit neuronal autophagy by targeting autophagy-related gene 7(Atg7), thereby reducing neuronal apoptosis induced by OGD [36]. In in vitro OGD primary neuron model and in vivo mouse MCAO model, Scientist found that adipose-derived mesenchymal stem cells, Mir-25-3p in exosomes secreted by ADMSC inhibit autophagy flux in damaged neurons after ischemic stroke by downregulating p53-BNIP3 signaling. In pathway, in thus in inducing in neuroprotective effect [37,38].
Enhance Neurite Remodelling
After ischemic stroke, many neurons die and denervate. Promoting neurite outgrowth and establishing new synaptic connections is the basis for a gradual recovery of nerve function. Scientist found that the expression level of Mir-133b in exosomes increased after multipotent mesenchymal stem cells (MSC) were exposed to rat ischemic brain tissue extracts [39]. Mir-133b was transferred into neurons and astrocytes by exosomes, and transfected Mir-133b significantly increased the number and length of neurite branches. In vivo studies, the team further found that Mir- 133b carried by MSC exosomes inhibited connective tissue growth factors in astrocytes, inhibited the expression of CTGF and Ras homologous family member A(RhoA) in the ischemic border zone, and inhibited the release of exosomes from astrocytes that promote neurite outgrowth. It significantly enhances axonal plasticity after ischemic stroke and promotes synaptic remodelling in the ischemic border area [40,41]. Scientist also found that MSC exosomes rich in Mir-17-92 cluster could activate the PI3K/AKT/mTOR signaling pathway by inhibiting the expression of phosphatase and tensin homolog (PTEN) [42]. Enhance the extension of axons after cerebral ischemia and promote the recovery of neurological function.
Promote Myelination
Oligodendrocytes are myelinating cells in the central nervous system and are vulnerable to injury during ischemic stroke. Although neuronal plasticity causes brain tissue to undergo a large amount of activity-dependent functional reorganization, leading to partial recovery of neurological function in patients, myelin repair is essential for the normal function and reorganization of neuronal networks [43]. Mir-134 in BMSC-derived exosomes inhibits apoptosis of rat oligodendrocytes by negatively regulating caspase-8, thus exerting neuroprotective effects [44]. Scientist found that MSC exosomes rich in Mir-17-92 cluster activates the PI3K/AKT/mTOR signaling pathway by inhibiting PTEN, thereby enhancing myelination and promoting the nervous system recovery after stroke [42]. Mir-126 in mouse brain endothelial exosomes can also improve neurocognitive function in type 2 diabetic mice with stroke by increasing myelin density [45]. However, the specific mechanism is not completely clear and needs to be further studied.
Promote Neurogenesis
The injury caused by a stroke can cause neurogenesis of ischemic penumbra in the infarct area. However, this endogenous neurogenesis process is slow, so improving its neurogenesis is a promising treatment for ischemic stroke [46]. Histone deacetylase 6(HDAC6) is a member of the Histone deacetylase family, which plays an important role in chromosome structure modification and gene expression regulation. Inhibition of HDAC6 after ischemic stroke can significantly promote neurogenesis and alleviate neurological deficits [47]. researcher found that Mir-26a in exosomes derived from human urine-derived stem cells could promote the proliferation of neural stem cells and neuronal differentiation by inhibiting HDAC6, thus improving the neurological deficit after ischemic stroke [48]. In addition, Scientist found that ADSCderived exosomes overexpressing Mir-126 promoted neurogenesis by increasing the expression of biarticular proteins (a sign of migrating neuroblasts) in the periinfarct region, thereby promoting neurological recovery after ischemic stroke [23].
Promote Angiogenesis
After ischemic stroke, the promotion of angiogenesis contributes to the recovery of neurological function and improves the survival rate of stroke patients [49]. Studies have shown that Mir-181b-5p in ADSC-derived exosomes promotes the migration and angiogenesis of brain microvascular endothelial cells after OGD by inhibiting transient receptor potential melastatin 7(TRPM7). Therefore, it has a protective effect after ischemic stroke [50- 52]. In addition, Mir-126 carried by ADSC exosomes can promote angiogenesis by upregulation of the expression of vWF, a marker of cerebrovascular endothelial cells in the peripheral area of infarction, and then improve neurological function recovery after ischemic stroke [23]. Interleukin-4 (IL-4) can differentiate BV2 microglia into M2 phenotype. Compared with the control group, the expression of Mir-26a in exosomes secreted by M2 BV2 cells is higher, which can improve the injury caused by ischemic stroke by promoting angiogenesis [53,54]. Scientist found that endothelial progenitor cells are rich in Mir-126. Exosomes released by EPC can increase cerebral blood flow and cerebrovascular density by down-regulating c-caspasE3 and up-regulating vascular endothelial growth factor receptor 2(VEGFR2) [55-57]. It promotes angiogenesis and neurogenesis, which is beneficial to the recovery of neurological function in diabetic ischemic stroke mice.
ENGINEERED EXOSOMAL MIRNA TARGETED DELIVERY FOR THE HEALING OF ISCHEMIC STROKE
In conclusion, miRNAs in exosomes have good potential in treating ischemic stroke, which provides the experimental basis for their clinical application in treating ischemic stroke. However, although exosomes can cross the BBB, the brain targeting ability of natural exosomes produced by cells is insufficient, which limits their clinical application [58-60]. Studies have found that modification of exosome membrane can improve its targeting ability [61]. Studies have found that exosomes binding RGDyk cyclic peptide could bind integrin αvβ3 of the BBB and deliver Mir-210- loaded exosomes to ischemic brain tissue [60-62]. To promote the expression of vascular endothelial growth factor (VEGF) in ischemic stroke lesions and improve angiogenesis after ischemic stroke. In addition, rabies virus glycoprotein (RVG) and lysosome-associated membrane glycoprotein 2B (Lamp2b) can bind to the acetylcholine receptor of the blood-brain barrier and effectively deliver Mir- 124 to the infarct site of ischemic stroke. Thus, the neurogenesis of neural precursor cells is promoted, and the prognosis of stroke is improved [63-66]. Therefore, the in-depth study of engineered exosomes may provide new strategies for treating ischemic stroke.
SUMMARY AND PROSPECT
Patients with ischemic stroke are often accompanied by neurological dysfunction, which seriously affects their quality of daily life and brings a huge burden to the family and society. There is currently no effective treatment for ischemic stroke patients who have exceeded the treatment time window, so new treatments need to be found. miRNAs in exosomes can be delivered to recipient cells and thus affect the function of recipient cells. Meanwhile, the exosomes coated with them have good compatibility, easily cross the blood-brain barrier, and can be modified by engineering. Therefore, exosomal miRNAs are expected to be effective therapeutic targets for ischemic stroke. However, although several studies have demonstrated the great potential of exosomal miRNAs in the treatment of ischemic stroke, the optimal cellular source of exosomes has not been identified, and the mechanism by which exosomal miRNAs exert their protective effect on ischemic stroke is not fully understood. At the same time, the research of exosomes in the treatment of ischemic stroke is still in the early stage, and large-scale and multi-center clinical trials are needed to ensure the safety and efficacy of exosomes before clinical application. Although facing many problems, using miRNA in exosomes for treating ischemic stroke still holds great promise. It is expected that the further development of exosome research may become a new direction of gene therapy for ischemic stroke.
REFERENCES
- Feigin VL, Stark BA, Johnson CO, Roth GA (2021) Global, regional and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 20(10): 795-820.
- Qingfeng Ma, Rui Li, Lijun W, Peng Y, Yuan W, et al. (2021) Temporal trend and attributable risk factors of stroke burden in China, 1990- 2019: an analysis for the global burden of disease study 2019. Lancet Public Health 6(12): e897-e906.
- Papanagiotou P, White CJ (2016) Endovascular reperfusion strategies for acute stroke. JACC Cardiovasc Interv 9(4): 307-317.
- Wachter R, Groschel K (2018) Acute treatment and secondary prophylaxis of ischemic stroke: an excellent example for personalized medicine. Internist 59(3): 241-251.
- Meyer M, Juenemann M, Braun T, Schirotzeket I, Tanislav C, et al. (2020) Impaired cerebrovascular autoregulation in large vessel occlusive stroke after successful mechanical thrombectomy: a prospective cohort study. J Stroke Cerebrovasc Dis 29(3): 104596.
- Nisar T, Hanumanthu R, Khandelwal P (2019) Symptomatic intracerebral hemorrhage after intravenous thrombolysis: predictive factors and validation of prediction models. J Stroke Cerebrovasc Dis 28(11): 104360.
- Kalluri R, Lebleu VS (2020) The biology, function and bio-medical applications of exosomes. Science 367(6478): eaau6977.
- Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88: 487-514.
- Zhang J, Li S, Li L, Li M, Guo C, et al. (2015) Exosome and exosomal microRNA: tracking, sorting, and function. Genomics Proteomics Bioinformatics 13(1): 17-24.
- Nozohouri S, Vaidya B, Abbruscato TJ (2020) Exosomes in ischemic stroke. Curr Pharm Des 26(42): 5533-5545.
- Khan SU, Khan MU (2022) Extra chromosomal circular DNA: recent advances in research. Biomed Res Environ Sci 3(4): 445-452.
- Tiwari A, Mukherjee B, Dixit M (2018) MicroRNA key to angiogenesis regulation: miRNA biology and therapy. Curr Cancer Drug Targets 18(3): 266-277.
- Correia De SM, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M (2019) Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci 20(24): 6249.
- Chen L, Heikkinen L, Wang C, Yang Y, Sun H, et al. (2019) Trends in the development of miRNA bioinformatics tools. Brief Bioinform 20(5): 1836-1852.
- Chen CM, Chu TH, Chou CC, Chien CY, Wang JS, et al. (2021) Exosomederived microRNAs in oral squamous cell carcinomas impact disease prognosis. Oral Oncol 120(4): 105402.
- Cui GH, Zhu J, Wang YC, Wu J, Liu JR, et al. (2021) Effects of exosomal miR-NAs in the diagnosis and treatment of alzheimer’s disease. Mech Ageing Dev 200: 111593.
- Chen J, Chopp M (2018) Exosome therapy for stroke. Stroke 49(5): 1083- 1090.
- Lambertsen KL, Finsen B, Clausen BH (2019) Post-stroke inflammationtarget or tool for therapy? Acta Neuropathol 137(5): 693-714.
- Guofeng C, Guoliang C, Haichun Z, Zhe Z, Kai L, et al. (2021) Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther 12(1): 2.
- Khan SU, Khan MU (2021) The mechanism of mammalian mitochondrial quality control system. Journal of Chemistry and Nutritional Biochemistry 2(2): 59-69.
- Ai Z, Cheng C, Zhou L, Yin S, Wang L, et al. (2021) Bone marrow mesenchymal stem cells-derived extracellular vesicles carrying microRNA-221-3p protect against ischemic stroke via ATF3. Brain Res Bull 172: 220-228.
- Orihuela R, Mcpherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4): 649-665.
- Zhao Y, Gan Y, Xu G (2020) Exosomes from MSCs overex-pressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci 260: 118403.
- Khan SU, Khan MU, Kalsoom F, Khan MI, Gao S, et al. (2022) Mechanisms of gene regulation by histone degradation in adaptation of yeast: an overview of recent advances. Archives of Microbiology 204(5): 287.
- Jiang M, Wang H, Jin M, Yang X, Ji H, et al. (2018) Exosomes from miR-30d- 5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem 47(2): 864-878.
- Geng W, Tang H, Luo S, Lv Y, Liang D, et al. (2019) Exosomes from miRNA- 126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res 11(2): 780-792.
- Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, et al. (2021) Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/ TRAF6 signaling pathway after ischemic stroke. Aging 13(2): 3060- 3079.
- Khan S, Khan M (2022) Molecular developments in cell models of fatty liver disease. DYSONALife Science 3(1): 16-29.
- Liu Z, Chopp M (2016) Astrocytes, therapeutic targets for neuroprotection and neuro restoration in ischemic stroke. Prog Neuro-biol 144: 103-120.
- Deng Y, Chen D, Gao F, Lv H, Zhang G, et al. (2019) Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng 13: 71.
- Song H, Zhang X, Chen R, Miao J, Wang L, et al. (2019) Cortical neuronderived exosomal microRNA-181c-3p inhibits neuroinflammation by downregulating CXCL1 in astrocytes of a rat model with ischemic brain injury. Neuroimmunomodulation 26(5): 217-233.
- Khan SU (2021) Therapeutic application of genetically engineered ribosome-inactivating toxin proteins for cancer. J Biomed Res Environ Sci 2(12): 1216-1228.
- Radak D, Katsiki N, Resanovic I (2017) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 15(2): 115-122.
- Zhang Y, Liu J, Su M, Wang X, Xie C (2021) Exosomal microRNA-22-3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/ BMF axis. Stem Cell Res Ther 12(1): 111.
- Zhang G, Zhu Z, Wang H, Yu Y, Chen W, et al. (2020) Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J Adv Res 24: 435-445.
- Khan SU, Khan MU (2021) Review on gene regulation: DNA-protein and protein-protein interactions and their regulatory elements. Journal of Chemistry and Nutritional Biochemistry 2(2): 35-45.
- Song Y, Li Z, He T, Qu M, Jiang L, et al. (2019) M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9(10): 2910-2923.
- Zhang D, Cai G, Liu K, Zhuang Z, Jia K, et al. (2021) Microglia exosomal miR-NA-137 attenuates ischemic brain injury through targeting Notch1. Aging 13(3): 4079-4095.
- Khan SU, Khan MU (2022) Treatment of diabetic muscular hyperplasia with natural and nutritional supplements. Global Journal of Biotechnology and Biomaterial Science 8(1): 001-008.
- Wu W, Liu J, Yang C (2020) Astrocyte-derived exosome-trans-ported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-kappaB/MAPK pathways. Brain Res Bull 163: 84-94.
- Shi Q, Cheng Q, Chen C (2021) The role of autophagy in the pathogenesis of ischemic stroke. Curr Neuropharmacol 19(5): 629-640.
- Pei X, Li Y, Zhu L, Zhou Z (2019) Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp Cell Res 382(2): 111474.
- Pei X, Li Y, Zhu L, Zhou Z (2020) Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle 19(8): 906-917.
- Kuang Y, Zheng X, Zhang L, Ai X, Venkataramani V, et al. (2020) Adiposederived mesenchymal stem cells reduce autophagy in stroke mice by extra-cellular vesicle transfer of miR-25. J Extracell Vesicles 10(1): e12024.
- Khan SU, Khan MU (2021) Recent developments and applications of single-cell RNA sequencing technology in cell classification. J Biomed Res Environ Sci 2(12): 1283-1290.
- Shen L H, Xin H, Li Y, Zhang RL, Cui Y, et al. (2011) Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodelling after stroke in mice. Stroke 42(2): 459-464.
- Xin H, Li Y, Buller B, Katakowski M, zhang Y, et al. (2012) Exosomemediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7): 1556-1564.
- Xin H, Li Y, Liu Z, Wang X, Shang X, et al. (2013) MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosomeenriched extracellular particles. Stem Cells 31(12): 2737-2746.
- Xin H, Wang F, Li Y, Lu QE, Cheung WL, et al. (2017) Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant 26(2): 243-257.
- Xin H, Liu Z, Buller B, Li Y, Golembieski W, et al. (2021) MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodelling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab 41(5): 1131-1144.
- Jia W, Kamen Y, Pivonkova H, Káradóttir RT (2019) Neuronal activitydependent myelin repair after stroke. Neurosci Lett 703: 139-144.
- Khan SU, Khan MU (2022) The role of amino acid metabolic reprogramming in tumor development and immunotherapy. Biochemistry and Molecular Biology 7(1): 6-12.
- Xiao Y, Geng F, Wang G, Li X, Zhu J, et al. (2018) Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem 120(2): 2109-2118.
- Khan SU, Khan MU (2022) Advances in innate immune memory of macrophages. Explor Immunol 2: 428-441.
- Venkat P, Cui C, Chopp M, Zacharek A, Wang F, et al. (2019) MiR- 126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke 50(10): 2865-2874.
- Marques BL, Carvalho GA, Freitas EMM, Chiareli RA, Barbosa TG, et al. (2019) The role of neurogenesis in neurorepair after ischemic stroke. Semin Cell Dev Biol 95: 98-110.
- Wang Z, Leng Y, Wang J, Liao HM, Bergman J, et al. (2016) Tubastatin A, an HDAC6 inhibitor, alleviates stroke-induced brain infarction and functional deficits: potential roles of α-tubulin acetylation and FGF-21 up-regulation. Sci Rep 6: 19626.
- Ling X, Zhang G, Xia Y, Zhu Q, Zhang J, et al. (2020) Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J Cell Mol Med 24(1): 640-654.
- Sun P, Zhang K, Hassan SH, Zhang X, Tang X, et al. (2020) Endotheliumtargeted deletion of microRNA-15a/16-1 promotes poststroke angiogenesis and improves long-term neurological recovery. Circ Res 126(8): 1040-1057.
- Yang Y, Cai Y, Zhang Y, Liu J, Xu Z (2018) Exosomes secreted by adiposederived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through microRNA-181b/TRPM7 axis. J Mol Neurosci 65(1): 74-83.
- Tian Y, Zhu P, Liu S, Jin Z, Li D, et al. (2019) IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv Clin Exp Med 28(4): 421-430.
- Wang J, Chen S, Zhang W, Chen Y, Bihl JC (2020) Exosomes from miRNA- 126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci Ther 26(12): 1255-1265.
- Tian T, Zhang HX, He CP (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150: 137-149.
- Salunkhe S, Dheeraj, Basak M, Chitkara D, Mittal A (2020) Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release 326: 599-614.
- Zhang H, Wu J, Wu J, Qi F, Jingchao Z, et al. (2019) Exosome-mediated targeted de-livery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology 17(1): 29.
- Yang J, Zhang X, Chen X, Wang L, Yang G (2017) Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7: 278-287.
Article Type
Review Article
Publication history
Received Date: August 18, 2022
Published: October 19, 2022
Address for correspondence
Rabia Ahmad, Department of Medicine and Health Sciences, University Sains Islam Malaysia, Malaysia
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Wirda ZMR, Asral F, Ahmad A, Rabia A. Exosomal miRNA Research Technology for Combating Ischemic Stroke. 2022- 4(5) OAJBS.ID.000502.
Figure 1: Different cell-derived exosomal miRNAs inhibit apoptosis and autophagy by acting on target genes, promote neurite remodeling and neurogenesis, and exert neuroprotective effects after ischemic stroke. B: miRNAs in MSCS exosomes play a protective role by promoting myelination. C: miRNAs in MSCS exosomes can reduce neuroinflammation and secondary nerve injury by inducing microglia to transform from M1 phenotype to M2 phenotype. D: MSC exosomes inhibit the inflammatory response of astrocytes through miRNA.
Figure 2: Neuroprotective mechanism of exosomal miRNA.