Breast Cancer Prognosis and Exposure to Hazardous Contaminants: An Observational Retrospective Study at Hermosillo, Sonora, Mexico
ABSTRACT
Breast cancer prognosis is related to genetic and non-genetic risk factors. Distinct models for risk prediction have been reported to evaluate the 5-year overall survival at the time of diagnosis. However, those models do not account for non-genetic risk factors, like residential exposure to hazards. In this work, the residential exposure to hazards (gas power plants (GPP), residual water sites (RWS), hazardous air pollutants (HAPs)) has been included for assessing prognosis. We hypothesize that there is a statistically significant association between a 5-year breast cancer prognosis and residential exposure to hazards at Hermosillo city.
The main findings include the association between 5-year prognosis and residential exposure to hazards in Hermosillo residents. Association models developed included, in addition to hazard exposure, the variables of age, high cholesterol and triglycerides, previous cancer(s), diabetes mellitus type 2, menopausal status, and the 5-year prognosis as the response variable. CMH test included the variables of residential exposure and 5-year prognosis in combination with one of the remaining risk factors. Non-significant models developed included hazard exposure, and the variables of familiar history of cancer, presence of chronic illness(es), presence of elements of metabolic syndrome, hypertension, elevated HDL-cholesterol, and LDL-cholesterol.
The conclusions of this work are that the associations between residential exposure to hazards, in combination with other variables (age, previous cancer(s), diabetes mellitus type 2, and menopausal status), predict for a poor 5-year prognosis. Models including the factors of older age, the presence of previous cancer(s), and postmenopausal status; predict a poorer 5-year prognosis (overall survival of 69 % or less).
KEYWORDS
Breast cancer prognosis; Environmental hazards; Observational studies
ABBREVIATIONS:
HAPs: Hazardous Air Pollutants; RWS: Residual Water Sites; GPP: Gas Power Plants
INTRODUCTION
Breast cancer is a heterogeneous disease characterized by the development of a malignancy in the mammary tissue Turashvili [1]. The prognosis of breast cancer varies person-to-person, as it depends on the stage of the disease at the time of diagnosis, the tumor-node-metastasis (TNM) classification, the molecular subtype, hormonal and Ki-67 status, and tumor grade, among others Kamranzadeh et al. [2]; Hirata et al. [3]; Hennigs et al. [4]. There are distinct risk assessment models for breast cancer, like BOADICEA, IBIS, the Gail model, and Prediction online tool Jacobi [5]; Quante [6]; Willoughby [7]; Gray [8]. In this work, the risk model includes the variable of residential exposure to hazards.
The use of those models for breast cancer prognosis is subject to the physician’s criteria for determining the best mode-of-action for the patient. However, there is no consensus regarding the best risk model for its use on prognosis Zhang [9]; Mühlbauer [10]. Modifiable risk factors, like nutrition and obesity, parity, and hormonal contraception, are often overlooked and unaccounted for in those risk assessment models. Another variable of importance is residential exposure to hazards. The long-term exposure to hazardous contaminants, a modifiable risk factor, may contribute to breast cancer risk. For this reason, we included this risk factor in our modelling.
Prior studies have evaluated the associations between breast cancer and residential exposure to hazards Garcia [11]; Liu [12]; Hart et al. [13]; Romieu [14]; Snedeker [15]; Aronson et al. [16]; Demers et al. [17]; Brody [18]; Aschengrau [19]; Gallagher [20]. A retrospective study in Cape Cod, Massachusetts, reports a moderate risk for breast cancer in those women exposed to perchloroethylene (PCE) (90th percentile OR = 1.0 - 1.5, smoothing analysis OR = 1.3 - 2.0, both for a 0 to 19 - year latency assumptions). Moreover, longterm exposure to hazardous air pollutants (HAPs) reported an increased risk for invasive breast cancers in the California Teachers’ Study cohort. Some studies report weak to increased breast cancer risk Griffith [21]; Brophy et al. [22], while others found no association but to the development of congenital malformations Vrijheid [23]. The Nurses’ Health Study reports long-term exposure to HAPs is not related to an increased breast cancer risk, except for a chemical present in pesticides. These and other observational retrospective studies debate whether historical exposure to hazards may relate to breast cancer risk. More studies with distinct mathematical modeling approaches are needed to evaluate the association between hazards and breast cancer risk Villa-Guillen [24].
This observational retrospective study reports the 5-year breast cancer prognosis by physicians and evaluates its association with residential exposure to hazards at Hermosillo city, Sonora, Mexico. This geographic region is of interest given its high incidence of breast cancer INEGI [25], and its potential historical exposure to contaminants derived from industrial activities Duarte- Tagles [26]; CCA [27]; Sanchez-Osorio [28]; SINA [29]; CONAGUA [30]. Prior studies conducted by Castrezana-Campos targeted Hermosillo as one of 120 municipalities in Mexico with high breast cancer prevalence, contaminated wells, and potential HAPs’ exposure given the NOx and SO2 emissions by the industrial sector Castrezana-Campos [31].
The present work seeks to evaluate the association of residential exposure to hazards with a 5-year prognosis in a cohort of breast cancer patients. Medical oncologists used their expertise to predict the 5-year prognosis for each patient. Neighborhoods of Hermosillo city were classified based on their exposure to HAPs, RWS, and GPP. Statistical evaluated potential associations between hazard exposure and breast cancer prognosis. Our hypothesis is that a 5-year prognosis (good, intermediate, or poor overall survival) relates to hazard exposure in Hermosillo residents.
METHODS
Study Population
The present observational retrospective study collected clinical cases of female breast cancers from the period of 2013- 2019 at local hospitals in Hermosillo, Sonora, Mexico. Those hospitals were the following: Hospital General del Estado de Sonora Dr. Ernesto Ramos Bours (HGE), Centro Estatal de Oncología Dr. Ernesto Rivera Claisse (CEO), CIMA, Hospital San José, Clínica del Noroeste. REDCap platform Harris [32] stored clinical data and de-identified it using the Safe Harbor method, following national LFM [33]; DOF [34] and international HHS [35] regulations for the protection and privacy of human subjects. Breast cancer cases were from women residing at least 10 years in Hermosillo city. Inclusion criteria were the following: female breast cancers, current residents of Hermosillo, and ID-verifiable residential addresses registered at the National Electoral Institute (INE) of Mexico, or their medical insurance card. Exclusion criteria were the following: male breast cancers, cancers other than breast, former Hermosillo residents or foreigners, and non-ID-verifiable residential addresses at INE or their medical insurance card.
Breast Cancer Database
For this study, we created a breast cancer database for further statistical and medical analyses. Clinical data collected from physical files at the hospitals previously mentioned included the following variables:
Residential information: Database stored current and former neighborhood(s), and current and former zip code(s). For this study, we considered the oldest information of the patient (≥ 10 years old) Brender [36] given the minimal time-latency for tumor development. The present research did not collect private addresses to comply with national and international regulations for the protection and privacy of human subjects.
Breast cancer diagnosis and status: Clinical data included the following information at the time of diagnosis: cancer type by surgery, stage of breast cancer, tumor grade and size, and breast density by mammography and ultrasound (BI-RADS classification). Also, database included current treatment (chemotherapy, radiotherapy, radical mastectomy, quadrantectomy, port-a-cath, breast reconstruction, palliative care, other medical procedure), and current status of the patient (under treatment, survivor, relapse, metastasis, deceased). Clinical files older than January 1st, 2013, were not included in the study sample. Files beyond the year 2013 are not available given national regulations for data storage in Hermosillo (Article 32, General Law of Health, Mexico) SSA [37].
Non-modifiable risk factors: Clinical data included information of non-modifiable risk factors, like age at the time of diagnosis, age at menarche, menopausal status (regular menses; irregular menses; perimenopausal; menopausal; postmenopausal; non-specified), personal history of cancer (breast cancer; other cancer(s); no prior cancer(s); non-specified), familiar history of cancer (first-line relatives (mother, father, grandparents, daughter, son); second-line relatives (siblings, aunt, uncle, cousin); no relatives with cancer; non-specified and prior chronic illnesses other than neoplasia (any chronic illness; hypothyroidism; none; unknown). Menopausal status comprised women onto three categories: premenopausal, perimenopausal, and postmenopausal. Premenopausal included women with regular and irregular menses. Postmenopausal included women diagnosed at the menopause or stated as postmenopausal. Race and ethnicity were not collected, as Hermosillo is a city constituted solely by Hispanics, considering this location with a homogenous population.
Modifiable risk factors: Database included information on established modifiable breast cancer risk factors. Those were alcohol consumption (abstainer or non-drinker, occasional consumer if ≤ 7 drinks per week, alcoholism or heavy consumer if > 7 drinks per week, non-specified) NIHS [38], smoking status (never smoker, current smoker, passive smoker, former smoker, non-specified) CDC [39], red meat consumption (no consumption, occasional consumption (1-2 times per week), moderate consumption (3-4 times per week), frequent consumption (5-7 times per week), non-specified), obesity (normal weight if BMI < 30 kg/m2, obesity if BMI ≥ 30 kg/m2, non-specified), exercise habits (sedentary, exercises frequently (daily exercise 30 min), non-specified), hormonal contraception (never user, yes (current or former user), non-specified), parity, hormonal replacement therapy (HRT) (yes, no, not applicable, non-specified), and presence of elements of metabolic syndrome as defined by the NCEP ATP III criteria (yes, no, non-specified) Huang [40].
Education, religion and occupation of the study cohort: Clinical database included the information of education, religion, and occupation at the time of breast cancer diagnosis. Education was that registered as the highest educational degree achieved by the patient (elementary school; junior high school; high school; technical career; university; non-specified; analphabetism). Religion was that manifested by the patient (Atheist; Catholic; Christian; Mormon; Jehovah Witness; Other; non-specified). Occupation was the employment of the patient (unemployed; employee; academia; engineer; accountant; own business; retired; other; non-specified).
Ethics Statement
The present research was reviewed and approved by the Institutional Review Boards (IRB) of the hospitals (HGE, CEO, CIMA, Hospital San José, Clínica del Noroeste) participating in this study. This study was also IRB-approved by the Secretary of Health of Sonora, and by the University of Sonora. The Mexican regulations, following the General Law of Health for Clinical Research (Article 17), approved the present research and classified it as without risk for the individual.
Hazard-Exposed Neighborhoods
Classification of hazard-exposed neighborhoods: INEGI Census 2010 provided the coordinates (latitude, longitude) of gas power plants (GPP), residual water sites (RWS), and industrial facilities producing chemical hazards INEGI [41]. The geospatial analysis considered neighborhood as the unit of analysis (ArcGIS version 10.7.1). Proximity analysis classified as hazard-exposed neighborhoods if their radial distance to GPPs was that of ≤ 4 km, or if their radial distance to RWS was that of ≤ 3 km. HAP-exposed neighborhoods were those with > 6 industrial facilities, and classified as highly-industrialized.
Industrial facilities producing chemical hazards: Industries considered as potential sources of chemical hazards were those classified by INEGI Census 2010 as follows: production of industrial laminates, furniture, coolers, and AC, dental laboratories, production of pesticides and fungicides, manufacturers of orthopedic materials, candle production, chemical laboratories, metal production, automobile assembly, manufacturers of agricultural products, technology machinery production and assembly, manufacturers of cleaning products, electronics production, and assembly, production of dyes and construction materials, iron foundries, printer suppliers, welding shops, production of toys and jewelry, manufacturers of chemical and clinical products, aquaculture products, polymers’ production, mechatronics, electrics, among othersh.
Breast Cancer Prognosis
Physicians provided the 5-year prognosis of the sample (n=297 breast cancer patients). The 5-year prognosis was that of expected overall survival post-diagnosis. A Predict online tool Gray [8] served to estimate the 5-year survival (%), and it was subject to each physician’s criteria for the evaluation of invasive breast cancers. The classifications for 5-year breast cancer prognosis were as follows: Good, if the expected overall survival was between 90 % -100 %; intermediate if it was between 70-89 %; and bad if the estimated 5-year survival was that of 69 % or less.
Physicians had access to the de-identified clinical data for each breast cancer case to evaluate the 5-year overall survival. Physicians had no access to the residential information, nor to the environmental exposure to hazards. The geospatial analyst had no access to the 5-year prognosis of the sample.
Statistical Analysis
Cochran-Mantel-Haenszel (CHM) test was used to evaluate the associations between modifiable and non-modifiable breast cancer risk factors, residential exposure to hazards, and 5-year breast cancer prognosis. Patients classified as residentially exposed to contaminants if reported to living in neighbourhoods GPP-exposed, RWS-exposed, or HAP-exposed. The statistical analysis was carried out using R version 3.4.2.
RESULTS
Characteristics of the Study Cohort
Medical oncologists analysed the clinical characteristics of patients at the time of diagnosis, and their status, for estimating the 5-year overall survival. Clinical data collected a total of n=306 patients. From those, nine cases were incomplete and excluded from the analysis. A total of n=297 patients comprised the study population.
Clinical characteristics of the study cohort at the time of breast cancer diagnosis: Table S1a describes the clinical characteristics of the study cohort. The most frequent breast cancer type, determined by surgery, was invasive ductal carcinoma (IDC), with 56.23 % of the cases. Secondly was ductal carcinoma in situ (DCIS) with 22.56 %. Metastatic breast cancer (MBC) was 3.03 % of the cases. Invasive lobular carcinoma (ILC), and lobular carcinoma in situ (LCIS) constituted 2.02 % and 1.01 %, respectively. Phyllodes tumors were 0.67 %, and other rare breast cancers were 2.02 %, giving a total of 2.69 % for rare breast cancers. Non-specified breast cancers comprised 12.46 %. This study sample did not include information for molecular breast cancer subtypes due to its unavailability on files.
In the study cohort, most of the cases did not report the stage at the time of diagnosis (33.33 %). 66.67 % had information regarding breast cancer stage. Late breast cancer stages (stage IV, IIIa, IIIb and IIIc) were 3.37 %, 6.73 %, 6.73 %, and 1.68 %of the cohort, respectively. Mid-stages (stage IIa, IIb) were 19.19 % and 9.43 % of the sample. Early stages (Ia, Ib) were 4.04 % and 0.34 % of the cases. Early-stage I non-specified (Stage I) was 10.44 % of the cohort. Stage 0 was only 2.02 % of the population. It is important to mention that the proportions of breast cancer types won’t match with those reported for the stage. The underreporting of TNM (no information for 45.45 %) and SBR (no information for 76.77 %) on files contribute to this mismatch.
Regarding tumor grade, 52.19 % of the cases had no information. 47.81 % had information on tumor grade. Most of them were that of grade II at the time of diagnosis (27.95 %). Grade III tumors represented 12.12 % of the cohort. Low-grade tumors (grade I) constituted the 7.74 %.
This analysis classified tumor size into three categories: size of x ≥ 2 cm, size of 2 cm < x < 5 cm, and size of x > 5 cm, where x represented the diameter of the tumor size. 43.43 % had no information on tumor size. The rest of the cohort (56.57 %) indicates that most of the tumors classified that of size x ≥ 2 cm (27.61 %), closely followed by tumors with a size of 2 cm < x < 5 cm (24.24 %). Tumors with a size of x > 5 cm represented 4.71 % of the sample.
Breast density was not reported by mammography in 61.62 % of the cases, leaving 38.38 %. Also, it was not reported by ultrasound in 78.79 % of the study sample, where the remaining cases were 21.21 %. The most frequent breast density by mammography (BI-RADS) was extremely dense breasts (20.88 %). Ultrasound shows the same, with the highest frequency classified as class D (15.15 %). Mammography and ultrasound provide similar percent for heterogeneously dense breasts, with 2.69 % and 3.03 %, respectively. For medium-breast densities, mammography and ultrasound have discrepancies. Breasts classified with scattered fibro glandular density were 7.41 % of the cases by mammography, whereas ultrasound indicates a 2.02 %. On the same line, mammography and ultrasound have discrepancies for low-dense breasts. Breasts classified as almost entirely fatty by mammography comprise the 7.41 %, whereas by ultrasound entails the 1.01 % of the study population.
Clinical characteristics of the study cohort at the status of the disease: Table S1a summarizes the variables collected for the status of the patient. Those are medical treatments, where 43.10 % of the patients received chemotherapy, 46.46 % had quadrantectomy, 39.73 % had a radical mastectomy, 29.97 % received radiotherapy, and 7.07 % received palliative care. Only 6.40 % of the patients underwent breast reconstruction, and 5.39 % were initiating treatment and had placement of a port-a-catheter. Other medical procedures constituted 37.37 % of the treatments (breast biopsies, grammagraphy) performed for the study cohort. Patients received more than one medical treatment.
The current status of the study cohort indicated the majority were under treatment (77.78 %), whereas 10.10 % reported as defunctions. Patients with metastasis represented the 8.08 % of the sample, and 3.03 % of the patients reported a relapse. Breast cancer survivors represented the smallest group, with 1.01 % of the study cohort.
Non-modifiable breast cancer risk factors: The database collected several non-modifiable risk factors from n=297 patients. Those were age at the time of diagnosis, personal history of cancer, familiar history of cancer, and prior chronic illnesses other than neoplasia (Table S1b).
The mean age of the study cohort was 55.04 years old, with a median of 54 years old. Personal history of cancer(s) indicates the majority had no prior neoplasia (83.50 %), while 13.13 % had breast cancer. 2.02 % reported cancers other than mammary tumors, and 2.02 % had no information.
The familiar history of cancer for this cohort was not available for 18.86 %. Information on familiar history was available for 81.14 % of the study population. A considerable proportion has a secondline relative (36.03 %) (includes having a brother, sister, cousin, aunt, or uncle with cancer). Patients with first-line relatives were that of 29.97 % (includes having a son, daughter, mother, father, or grandparent with cancer). 28.62 % had no family with cancer. Data on age at menarche was not available on 30.30 % of files. From the remaining 69.70 %, the mean age at menarche was 12.96 years old, with a median of 13 years old.
Menopausal status data was not available for 8.08 % of files. 91.92 % of files had this information. Most of the study subjects classified as postmenopausal (55.56 %). 11.78 % of women classified as perimenopausal, while 17.51 % reported regular menses at the time of diagnosis. 4.04 % of women were menopausal, and 3.03 % indicated irregular menses.
Additionally, the database collected information regarding prior chronic illnesses other than neoplasia. Only 2.02 % had no information. 97.98 % had records regarding chronic diseases. The majority reported no prior illnesses (60.61 %), while 37.37 % presented a chronic disease before the diagnosis. 9.43 % of the cohort had hypothyroidism.
Modifiable breast cancer risk factors: The present study collected information regarding modifiable risk factors related to breast cancer development (Table S1c). Those were alcohol consumption, smoking status, red meat consumption, obesity, exercise habits, use of hormonal contraception, parity, age at menarche, menopausal status, hormonal replacement therapy (HRT), and presence of elements of metabolic syndrome (defined as the NCEP ATP III criteria).
23.57 % of the cohort had no information regarding alcohol consumption. Data was available for 76.43 %. Most of the patients were non-consumers (58.92 %). Occasional drinkers represented 15.82 % of the sample, and heavy drinkers were only 1.68 %.
For smoking status, 20.88 % of the files had no information. Smoking status was available for 79.12 % of the sample. Most of the cohort classified as never-smokers (59.26 %). Current smokers represented 12.12 % of the study group. Former smokers comprised 5.39 % of the population, and passive smokers represented 2.36 %.
Red meat consumption data was not available for 74.75 % of the sample. Information was available for 25.25 %. 15.82 % consume red meat occasionally (average 1-2 times per week). 8.08 % reported a moderate consumption of red meat (average 3-4 times per week). Only 0.67 % reported frequent red meat consumption (average 5-7 times per week). Vegans or non-red meat consumers were 0.67 % of the study sample.
Information regarding obesity was not available for 1.68 % of the sample. Data was available for 98.32 % of the cohort. Obesity, here defined as BMI ≥ 30 kg/m2, was present in 39.39 % of the cohort. Most of the study subjects classified as that of normal weight (58.92 %) at the time of diagnosis.
Data about exercise habits was not available for 58.25 % of the cohort, leaving a 41.75 %. Regarding exercise habits, 35.02 % of subjects were sedentary. This percent is similar to that for obese patients. A small proportion of subjects (6.73 %) reported frequent physical activity, here defined as 30 min of exercise per day.
Data on hormonal contraception use was not available for 54.88 % of the cohort, leaving 45.12 %. 30.64 % reported no prior use of hormonal contraception, while 14.48 % indicated were current or former users.
Information about parity was not available for 2.02 %, whereas 97.98 % of files had this data. Most of the subjects classified as multiparous (70.03 %) here defined as having more than one live birth of a child. Parous women (only one live birth) were 12.79 % of the sample. Nulliparous women were that of 15.15 %.
In terms of HRT, information was not available on files for 61.95 % of the population. From the rest (38.05 %), a considerable proportion of women reported HRT as not applicable, given their menopausal status (23.57 %). For those women considered as menopausal or postmenopausal, 11.78 % of them were non-HRTusers.
0.34 % of files had no information about the presence of elements of metabolic syndrome, leaving 99.66 % with available data. From those, 70.71 % of the cohort had at least one element of metabolic syndrome. In contrast, 28.96 % had no elements of metabolic syndrome.
Education, religion, and occupation of the study cohort: Database compiled information regarding the highest educational level achieved by the patient, religion, and current occupation [Table S1d].
Information about the education was not available for 21.21 % of the files. The remaining 78.79 % was as follows: a considerable proportion has university studies (27.95 %), 2.36 % had a technical career, 9.09 % had a high school, 22.56 % had a junior high school, 15.15 % had an elementary school, and 1.68 % were an alphabet.
Religious beliefs were not on 4.04 % of files, leaving 95.96 % with available information. Most of the study cohort is Catholic (81.82 %). Christians represented 10.77 % of the population. Atheists were that of 1.68 %. Jehovah’s Witnesses comprise only 0.67 % of the population, and 0.34 % were Mormons. Other religions or personal beliefs represented 0.67 %.
According to occupation data, 2.36 % had no information, whereas occupation was on 97.64 % of the files. The majority of this cohort was unemployed (68.69 %). 14.14 % of the population reported being formal employees. Those women owning a business were that of 6.06 %, whereas those employed in academia represented 4.04 %. Engineers were that of 1.01 %, and accountants were that of 0.67 %. Retired were that of 2.69 % of the cohort, while informal occupations comprised 0.34 %.
Qualitative Categories for a 5-Year Breast Cancer Prognosis in the Study Cohort
Medical oncologists and radio oncologists evaluated n=297 breast cancer cases according to clinical data captured and deidentified in REDCap. The criteria were qualitative and based on their medical experience with breast cancer cases. Physicians used Predict online tool as an aid for estimating the 5-year overall survival for invasive breast cancers. This tool, however, did not substitute physicians’ expertise. The physicians had the final decision on categorizing breast cancer prognosis. A total of n=111 (37.37 %) cases classified with a good prognosis, estimating a 5-year survival of 90-100 % post-diagnosis. 89 (29.97 %) patients classified with an intermediate prognosis, with an expected survival between 70-89 %. Also, n=97 (32.66 %) cases classified with a bad 5-year prognosis, where the overall survival is 69 % or less.
Associations between Hazard Exposure, Breast Cancer Risk Factors, and 5-Year Prognosis
CMH test evaluated the associations between breast cancer risk factors and 5-year prognosis for the study cohort. The models are depicted in Table 1. To avoid bias, the physicians and the geospatial analyst were double-blinded. Full data was disclosed only to the research statistician. Each model used to analyze associations is described in detail in further sections.
Models considered three variables, where all of them include the level of exposure (explicative variable) and prognosis (the outcome variable). The second explicative variable is that of a risk factor (age, high cholesterol, high triglycerides, previous cancer(s), diabetes mellitus type 2, menopausal status). Residential exposure to hazards was in all models to evaluate because of its potential association with a 5-year prognosis (Table 1).
The model with four variables also considered prognosis as the outcome variable. Residential exposure to hazards was included as an explicative variable along with the remaining variables of age, and previous cancer(s).
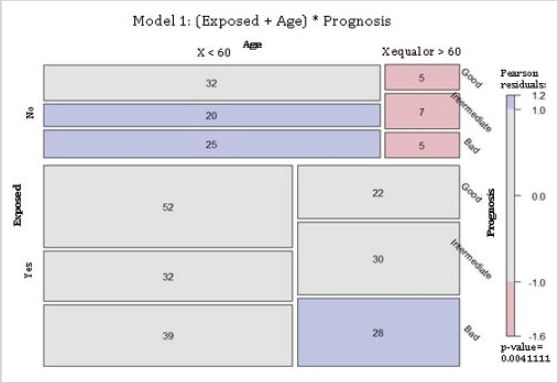
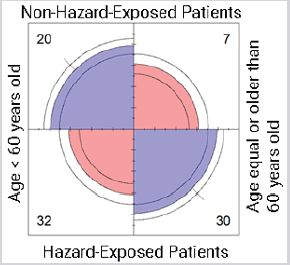
Residential exposure to hazards, age and 5-year breast cancer prognosis: Model 1 (Table 1) evaluated the associations between 5-year breast cancer prognosis, hazard exposure, and age. The null hypothesis was that, given a 5-year prognosis, residential exposure and age were independent. The null hypothesis was rejected, meaning that, given a 5-year prognosis, residential exposure and age were dependent in this cohort. Mosaic plot depicts Model 1, where boxes indicate deviation of the expected values. Red boxes indicate the value is lower than that expected. Blue boxes indicate the value is higher than that expected. Gray boxes indicate the value is approximately equal to that expected. The non-exposed group presents more cases younger than 60 years old than that expected for an intermediate and a bad 5-year prognosis. Also, there were fewer patients equal or older than 60 years old than that expected for all categories of 5-year prognosis. For the hazard-exposed group, there were more breast cancer cases than that expected for women equal or older than 60 years old with a bad 5-year prognosis (Figure 1).
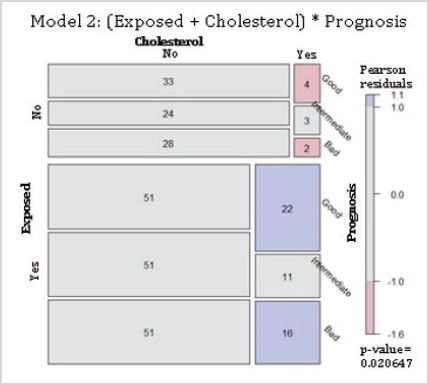
Residential exposure to hazards, high cholesterol and 5-year breast cancer prognosis: The model 2 proposed that, given a 5-year prognosis, hazard exposure and high cholesterol were independent (Table 1). The null hypothesis was rejected, meaning that, given a 5-year breast cancer prognosis, the variables of residential exposure and high cholesterol were associated. Mosaic plot indicated there were fewer cases than that expected for those non-hazard-exposed with hypercholesterolemia predicted with both good and bad prognoses. For the hazard-exposed group, there were more cases than expected for women with high cholesterol with good and bad prognosis (Figure 2).
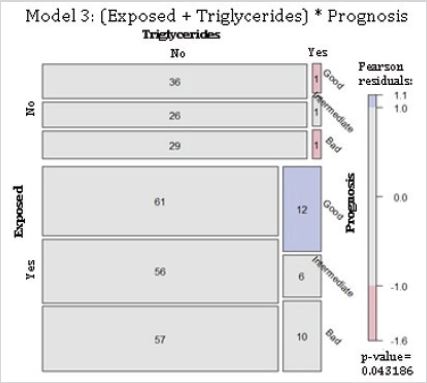
Residential exposure to hazards, high triglycerides and 5-year breast cancer prognosis: Model 3 proposed that, given a 5-year prognosis, residential exposures to hazards and high triglycerides were independent (Table 1). Results rejected this null hypothesis. These results mean that, for a given 5-year prognosis, there is an association between hazard exposure and high triglycerides. The mosaic plot depicts the dependence between the variables. There were fewer cases than that expected for women with high triglycerides, non-exposed to contaminants, and with both good and bad prognoses. For the hazard-exposed group, there were more cases than expected for women with a good prognosis and high triglycerides (Figure 3).
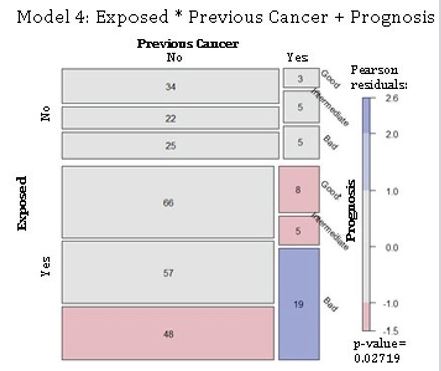
Residential exposure to hazards, previous cancer(s) and 5-year breast cancer prognosis: The model 4 proposed that a 5-year prognosis is jointly independent of residential exposure to contaminants and previous cancer(s) (Table 1). The null hypothesis was rejected, where a 5-year prognosis is not jointly independent of those explicative variables. The number of cases was approximately the expected value for non-exposed women with and without previous cancer(s). However, the exposed group showed there were fewer cases for women with no prior cancer(s) and with a bad 5-year prognosis. Also, exposed women to hazards with previous cancer(s) presented lower numbers than that expected for good and intermediate prognoses. Moreover, model 4 showed more cases than that expected for exposed women with prior cancer(s) with a bad prognosis (Figure 4). Those results confirmed the rejection of the null hypothesis. The 5-year prognosis was not independent for a combination of exposure to hazards and previous cancer(s).
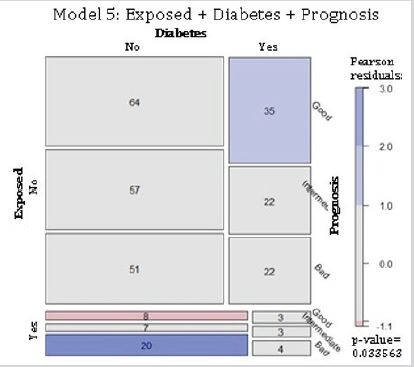
Residential exposure to hazards, diabetes and 5-year breast cancer prognosis: The model 5 evaluated the complete independence between residential exposure to hazards, diabetes mellitus type 2, and 5-year breast cancer prognosis. The null hypothesis was that all variables were independent of each other (Table 1). Findings indicate there were more cases than that expected for non-exposed diabetic women with a good prognosis. The exposed, non-diabetic group presented fewer cases for a good prognosis, but more than the estimated for those with a bad prognosis (Figure 5). Results confirmed the rejection of the null hypothesis, indicating that the variables of residential exposure to hazards, diabetes mellitus type 2, and 5-year breast cancer prognosis were not complete independent for this cohort.
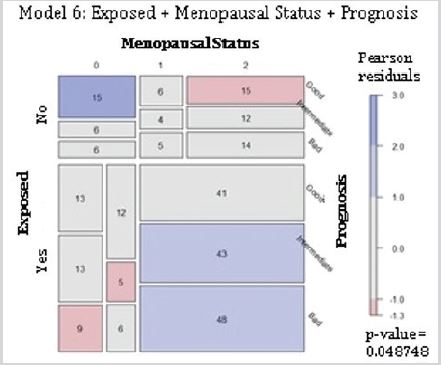
Residential exposure to hazards, menopausal status and 5-year breast cancer prognosis: The null hypothesis established that the variables of hazard exposure, menopausal status, and 5-year prognosis were completely independent (Table 1). The null hypothesis was rejected, where there were more cases than the expected for premenopausal women not exposed to contaminants and with a good prognosis. Also, there were fewer cases than expected for exposed postmenopausal women with a good prognosis. For the exposed group, there were fewer cases than expected for premenopausal women with intermediate and bad prognoses. Moreover, the cases registered for peri- and postmenopausal women exposed to contaminants were more than the expected for an intermediate and a bad prognosis (Figure 6). Those findings confirmed the rejection of the null hypothesis. For this cohort, the variables of hazard exposure, menopausal status, and 5-year breast cancer prognosis were not independent of each other.
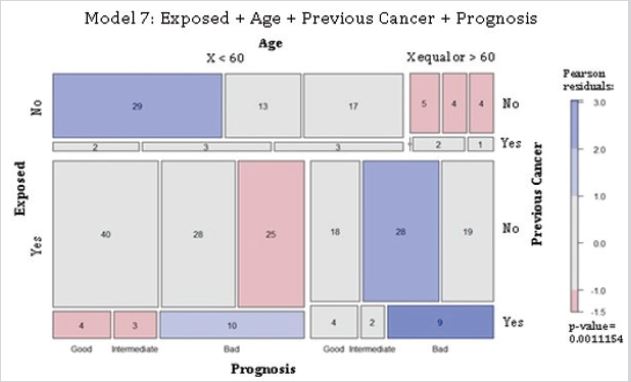
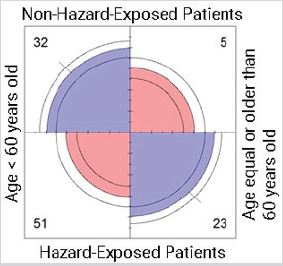
Residential exposure to hazards, age, previous cancer and 5-year breast cancer prognosis: Model 7 proposed complete independence of the variables of residential exposure to hazards, age, previous cancer, and 5-year prognosis for the study cohort (Table 1). Findings rejected the null hypothesis. The non-exposed group presented more cases than that expected for women younger than 60, without previous cancer(s), for good and intermediate prognoses. There were fewer cases than that expected for nonexposed women equal or older than 60 years old, without prior cancer(s), for all prognoses. The exposed women younger than 60 years old, and with previous cancer(s), presented more cases than that expected for a bad prognosis. Also, those women had fewer cases than the expected for those without previous cancer(s), and a bad prognosis. Additionally, that age group, with prior cancer(s), had fewer cases for good and intermediate prognoses. Exposed women with age equal or older than 60, and without previous cancer(s), had higher numbers than the expected for an intermediate prognosis. Moreover, that age group but with a cancer history had higher cases than that expected for a bad prognosis (Figure 7). Results confirmed the null hypothesis rejection, indicating that the variables of hazard exposure, age, previous cancer(s), and 5-year breast cancer prognosis were not independent of each other.
Non-significant associations between hazard exposure, potential breast cancer risk Factors, and 5-year prognosis: Models evaluated other associations of a 5-year breast cancer prognosis, hazard exposure, and other potential breast cancer risk factors (familiar history of cancer, presence of chronic illness, presence of elements of metabolic syndrome, hypertension, HDLcholesterol, and LDL-cholesterol). Table 2 depicts the models of those explicative variables with 5-year prognosis and hazard exposure. The null hypothesis was accepted, where the output indicated no lack of fit according to the deviance test statistic and Pearson (X2). The model of complete independence fitted the data. Findings indicate the variables proposed in each model were independent of each other, and as such, there was no further analysis.
DISCUSSION
This observational retrospective study evaluated associations between 5-year prognosis, residential exposure to hazards, and risk factors for breast cancer patients at Hermosillo city. Models evaluated the associations of distinct risk factors with the 5-year prognosis as the outcome variable. All models included residential exposure to hazards.
Models for 5-Year Prognosis, Hazard Exposure and Risk Factors
Models predicted a poor outcome (overall 5-year survival of 69 % or less) for those hazard-exposed patients with advanced age (equal or older than 60 years old), postmenopausal, or with previous cancer(s). Older patients who are hazard-exposed and with prior cancer(s) are particularly at higher risk for bad prognosis. Models predict better outcomes (overall 5-year survival of 90 – 100 %) for non-exposed younger patients (less than 60 years old), with no previous cancer(s), or with diabetes in comparison to that for hazard-exposed.
Models also suggested a 5-year prognosis was independent of a familiar history of cancer, prior chronic illness(es), hypertension, reduced HDL-cholesterol, elevated LDL-cholesterol, and the overall presence of elements of metabolic syndrome in hazard-exposed patients. Models evaluating high cholesterol or triglycerides in exposed patients, even though statistically significant, did not contribute to predicting a bad prognosis.
To the best of our knowledge, the present work is the first research conducted for estimating breast cancer prognosis in a cohort residentially exposed to hazards at Hermosillo city. Prior research suggested hazardous air pollutants as related to a higher breast cancer incidence in the California Teachers’ Study. Longterm exposure (10 to 19 years) to perchloroethylene (PCE) related to an increased rate for breast cancers in Cape Cod, Massachusetts. Other studies showed no association between breast cancer and environmental hazards. A cross-sectional analysis within the Nurses’ Health Study II found no relationship between breast cancer risk and historical exposure to HAPs. Drinking water contaminated by wastewater showed no relationship to an increased breast cancer burden in 824 women residing at cape cod, Massachusetts [Brody 2006]. Mathematical modeling in these and other retrospective studies consider annual exposure to hazards and thus may be a source for discrepancies in assessing hazard exposure as a breast cancer risk factor. Future works could include risk assessment and hazard exposure at Hermosillo using distinct mathematical models.
Modifiable and Non-Modifiable Risk Factors in the Study Cohort
Breast cancer risk assessment varies according to modifiable and non-modifiable risk factors Arthur [42]. Non-modifiable risk factors include tumor status at the time of diagnosis. Previous works suggest mortality rates increase with tumor size and with the number of positive lymph nodes Rakha [43]; Saadatmand [44]. Histological tumor grade indicates the degree of differentiation, where high-grade tumors (grade II or III) are poorly differentiated. Those tumors are more likely to spread and metastasize. The study cohort had a higher proportion of subjects with invasive breast cancers (56.23 %), invasion to lymph nodes (19.53 %), and grade II tumors (27.95 %). The frequency of Phyllodes tumors was higher in this cohort (0.67 %) than the average reported per 100 breast cancer cases, which is 0.3 % to 0.5 % Rowell [45]. According to this tumor status, a considerable proportion of the study population could be at a higher risk for a worse 5-year prognosis. Molecular profiling tests of tumors could aid in a better outcome prediction for breast cancer patients at Hermosillo city.
Observational investigations suggest certain chronic illnesses relate to cancer risk and a reduction in lifespan. A recent study in Taiwan found that chronic diseases (cardiovascular disease, diabetes, pulmonary disease, chronic kidney disease, and gouty arthritis) increased two-fold the cancer risk after 8 years of followup Tu [46]. Moreover, those cancer patients with a prior chronic illness had a four-fold increase in cancer deaths, with a lifespan reduction of 15.9 years in women. The presence of prior chronic illnesses in the study cohort (37.37 %) could indicate a reduction in lifespan in comparison to those cancer patients without chronic diseases before diagnosis (60.61 %). More prospective studies need to evaluate common chronic illnesses and their markers in Hermosillo residents considered at a higher risk for cancer development.
Breast density, on the other hand, is a modifiable risk factor where the highest density relates to a higher breast cancer risk Wang [47]. In this cohort, a considerable proportion of subjects had extremely dense breasts (20.88 % mammography, 15.15 % US). Most breast cancer cases were postmenopausal (55.56 %). The high breast density, along with postmenopausal status, may increase the risk for this study cohort. Prior studies suggest tumor aggressiveness in postmenopausal women with dense breasts Yaghjyan [48]. This behaviour may be like the present study population. Breast density can also relate to the familiar history of cancer for increased breast cancer risk. Duffy [49] suggested a higher risk for subjects with both high breast densities and a familiar history of breast cancer. 66 % of the cohort had relatives with cancer and could be at a higher risk in combination with high breast densities. Imaging analyses and genetic counselling could elucidate the potential role of those risk factors for Hermosillo residents.
Obesity is a well-known risk factor associated with breast cancer development and its recurrence. Obese breast cancer patients have a worse disease-free and overall survival in comparison to nonobese patients Lee [50]. Moreover, several investigations indicate obesity increases the risk for postmenopausal ER-positive breast cancers, with increased cancer mortality in comparison to their lean counterparts Picon-Ruiz [51]. A meta-analysis of 22 prospective studies associated pre-diagnosis physical activity with a 30 % lower cancer mortality for breast cancer survivors (HR = 0.73, 95 % CI, 0.54 – 0.98). Moderate physical activity post-diagnosis (approximately 2.5 hours per week) relates to a 33 % reduction in breast cancer mortality (HR = 0.59, 95 % CI, 0.45 - 0.78) Lahart [52]. In the study cohort at Hermosillo, an important proportion was obese (39.39 %) and sedentary (35.02 %) and could be at a higher risk for a poor 5-year prognosis. Nutritional assessment and trials aiming for exercise interventions could improve the outcomes for those subjects.
Metabolic syndrome is related to a higher breast cancer risk. An observational study found that 40 % of breast cancer patients had metabolic syndrome, where there was a positive association between metabolic syndrome and breast cancer risk (OR = 3.037, 95 % CI, 1.214 – 7.597) Wani [53]. A meta-analysis evaluated nine cohort studies, where metabolic syndrome relates to a higher risk of breast cancer recurrence (RR = 1.52, p = 0.02). Also, breast cancer patients with metabolic syndrome had an increased risk for cancer mortality (RR = 1.80, p < 0.001) Li [54]. Most of the study population of the present research had at least one element of metabolic syndrome (70.71 %), and this could increase the probability of a bad prognosis.
Meat consumption can also increase breast cancer risk. The Sister Study evaluated meat consumption in a group of breast cancer patients, and compared them with those consuming poultry, finding those with regular meat consumption had a higher risk for invasive breast cancers (HR = 1.23, ptrend = 0.01) Lo [55]. At Hermosillo, the study cohort reports red meat consumption in a considerable percentage of the subjects (24.57 %) and may increase their risk for a worse prognosis. Future analyses at Hermosillo’s cohort will include meat consumption and 5-year overall survival for breast cancer patients.
Exogenous hormones, either by hormonal contraception or HRT-usage, may increase breast cancer risk and contribute to a poorer outcome. A recent study among hormonal contraception users suggested an overall increase of 13 breast cancers per 100,000 person-years (95 % CI, 10 to 16) Morch [56]. Surprisingly, a considerable proportion of Hermosillo’s cohort are non-HRT users (11.78 %) and report no hormonal contraception (30.64 %). This behavior may relate to religious beliefs, as most of the study subjects manifested to be Catholics (81.82 %), where one of the precepts of this religion is to avoid the use of hormonal contraception or HRT CSA [57]. Non-users could have reduced exposure to exogenous hormones in comparison to HRT-users (2.69 %) or those using hormonal contraception (14.48 %), reducing their breast cancer risk. Future works will include a correspondence analysis between these modifiable risk factors and 5-breast cancer prognosis in women at Hermosillo with distinct religious beliefs.
Alcohol consumption and smoking status relate to higher risk and may contribute to a worse 5-year prognosis. The WECARE Study evaluated women with breast cancer and their risk for contralateral breast cancer (CBC), where those with alcohol consumption and cigarette smoking had an increased risk for CBC (RR = 1.62, 95% CI 1.24-2.11) Knight [58]. The contribution of those risk factors to breast cancer risk is not consistent through the literature. An observational study in young women found no association between breast cancer risk, smoking status, or alcohol intake. In contrast, moderate alcohol consumption (5 grams per day) related to a lower breast cancer risk (OR = 0.6, 95 % CI, 0.4 – 0.9) Adami [59]. The role of alcohol and smoking status for breast cancer risk is unclear for Hermosillo’s cohort. Most of the subjects did not consume alcohol (58.92 %) or were non-smokers (59.26 %). More prospective studies need to further assess the relationship between breast cancer risk, alcohol consumption, and smoking status at Hermosillo’s cohort.
Other risk factors associated with breast cancer are nulliparity and a low educational level. Even though parity relates to a reduced risk for breast cancer Kana [60]; Fortner [61]; Redondo [62], most of the study cohort had a child (82.82 %) where the protective role of this variable is questionable. Some studies associate lower educational attainment with a diagnosis at later tumor stages Liu [63]. Nonetheless, a significant percentage of this cohort was highly educated (high school, 9.09 %; university studies, 27.95 %). This high education may indicate a greater breast cancer awareness in the study population. Further analysis will be conducted at Hermosillo to evaluate the associations of those risk factors with 5-year overall survival.
Potential Bias and Confounders
Move-outs can confound the retrospective assessment of residential exposure to hazards. In this study, all patients had a minimal of 10 years of residence in a neighborhood classified as GPP-exposed (within 4 km of a gas power plant (GPP)), RWSexposed (within 3 km of a residual water site (RWS)), or HAPexposed (neighborhood with > 6 active industrial facilities). This research discarded move-outs, avoiding bias in terms of location.
The occupation can be a source of hazard exposure and could be a confounder of this study. For this reason, data collected the occupation of all patients in this cohort at the time of diagnosis. Most of the subjects reported themselves as unemployed (68.69 %), indicating that they spent most of their time at home. This group could be potentially increasing their timeframe for hazard exposure in comparison to women working outside of their houses. Further analyses will include the timeframe of residential exposure to hazards for Hermosillo’s residents with breast cancers.
LIMITATIONS AND STRENGTH
Limitations of this research are the small sample size, indicating the need for larger observational studies. The study cohort was constituted solely by Hispanics, and findings may not be transferable to other races or ethnicities. The absence of genetic information on clinical files impaired a further genetic assessment for the study cohort. Research derived from the present study may include genetic counseling and testing for germline mutations.
The present work has several strengths. One of them is the retrospective evaluation of residential exposure to contaminants using the neighborhood as the unit of analysis. Tumor latency requires a minimal of 10 years of hazard exposure. Data collection included the potential timeframe for tumor development, including residential information with a minimal of 10 years old. Doubleblinding enabled to avoid bias, as the physicians had no access to residential information, whereas the geospatial analyst had none to the patients’ medical records.
CONCLUSION
Breast cancer risk factors can influence the overall 5-year survival for a patient. In a small sample of patients residing at Hermosillo city, a poor prognosis (overall 5-year survival of 69 % or less) relates to residential exposure to hazards, along with age, previous cancer(s), and menopausal status. Interestingly, the models predict a higher proportion of bad prognoses for hazardexposed, non-diabetic cancer patients. Physicians need to ponder those risk factors for cancer patients considered as hazard-exposed at Hermosillo. Larger cohorts could confirm our findings.
ACKNOWLEDGEMENT
The first author of this research was the recipient of the Consortium Arizona-Mexico Arid Environments (CAZMEX) Award 2018 for Postdoctoral Stays. CAZMEX is formed by the University of Arizona and the Mexican Council of Science and Technology (CONACyT).
The authors want to thank all the institutions that collaborated in this work: University of Sonora, Clinica del Noroeste, Hospital San Jose, Hospital General del Estado de Sonora Dr. Ernesto Ramos Bours (HGE), Centro Estatal de Oncología Dr. Ernesto Rivera Claisse (CEO), CIMA, and INEGI at Hermosillo, Sonora, Mexico. Special thanks to Armando Yanez from INEGI, and to Shelene Tarazon from HGE, for their help in providing an important quantity of the data. We are very thankful to the teaching heads M.S. Maru Eugenia from Clinica del Noroeste, Dr. Carlos Gonzalez-Becuar from HGE, and Dr. Frankfer Urias from CEO, for their support in data acquisition. Special thanks to Tanyha Zepeda for her assistance with REDCap platform.
REFERENCES
- Turashvili G, Brogi E (2017) Tumor heterogeneity in breast cancer. Front Med (Lausanne) 4: 227.
- Kamranzadeh H, Ardekani RM, Kasaeian A, Sadighi S, Maghsudi S, et al. (2019) Association between Ki-67 expression and clinicopathological features in prognosis of breast cancer: A retrospective cohort study. J Res Med Sci 24: 30.
- Hirata BKB, Oda JMM, Guembarovski RL, Ariza CB, Coral de Oliveira CE, et al. (2014) Molecular markers for breast cancer: prediction on tumor behavior. Hindawi 12.
- Hennigs A, Riedel F, Gondos A, Sinn P, Schirmacher P, et al. (2016) Prognosis of breast cancer molecular subtypes in routine clinical care: A large prospective cohort study. BMC Cancer 16: 734.
- Jacobi CE, de Bock GH, Siegerink B, van Asperen CJ (2009) Differences and similarities in breast cancer risk assessment models in clinical practice: which model to choose? Breast Cancer Res Treat (2009) 115(2): 381-390.
- Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB (2012) Breast cancer risk assessment across the risk continuum: Genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res 14(6): R144.
- Willoughby A, Andreassen PR, Toland AE (2019) Genetic testing to guide risk-stratified screens for breast cancer. J Pers Med 9(1): 15.
- Gray E, Marti J, Brewster DH (2018) Independent validation of the PREDICT breast cancer prognosis prediction tool in 45,789 patients using Scottish Cancer Registry data. Br J Cancer 119: 808-814.
- Zhang Z, Bien J, Mori M, Jindal S, Bergan R (2019) A way forward for cancer prevention therapy: personalized risk assessment. Oncotarget 10(64): 6898-6912.
- Mühlbauer V, Berger-Höger B, Albrecht M (2019) Communicating prognosis to women with early breast cancer-overview of prediction tools and the development and pilot testing of a decision aid. BMC Health Serv Res 19: 171.
- Garcia E, Hurley S, Nelson DO, Hertz A, Reynolds P (2015) Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environ Health 14: 14.
- Liu R, Nelson DO, Hurley S, Hertz A, Reynolds P (2015) Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: the California Teachers Study. Epidemiology 26(3): 365-373.
- Hart JE, Bertrand KA, DuPre N, James P, Vieira VM, et al. (2018) Exposure to hazardous air pollutants and risk of incident breast cancer in the nurses’ health study II. Environ Health 17: 28.
- Romieu I, Hernandez-Avila M, Lazcano-Ponce E, Weber JP, Dewailly E (2000) Breast cancer, lactation history and serum organochlorines. Am J Epidemiol 152(4): 363-370.
- Snedeker SM (2001) Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin. Environ Health Perspect 109 (Suppl 1): 35-47.
- Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, et al. (2000) Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prevent 9(1): 55-63.
- Demers A, Ayotte P, Brisson J, Dodin S, Robert J, et al. (2000) Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations. Cancer Epidemiol Biomarkers Prevent 9: 161-166.
- Brody JG, Aschengrau A, McKelvey W, Swartz CH (2006) Breast cancer risk and drinking water contaminated by wastewater: A case control study. Environ Health 5: 28.
- Aschengrau A, Rogers S, Ozonoff D (2003) Perchloroethylenecontaminated drinking water and the risk of breast cancer: additional results from cape cod, Massachusetts, USA. Environ Health Perspect 111: 167-173.
- Gallagher LG, Vieira VM, Ozonoff D, Webster TF, Aschengrau A (2011) Risk of breast cancer following exposure to tetrachloroethylenecontaminated drinking water in cape cod, Massachusetts: reanalysis of a case-control study using a modified exposure assessment. Environ Health 10: 47.
- Griffith J, Duncan RC, Riggan WB, Pellom AC (1989) Cancer mortality in US counties with hazardous waste sites and ground water pollution. Arch Environ Health 44: 69-74.
- Brophy JT, Keith MM, Watterson A, Park R, Gilbertson M, et al. (2012) Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: A Canadian case-control study. Environ Health 11: 87.
- Vrijheid M (2000) Health effects of residence near hazardous waste landfill sites: A review of epidemiologic literature. Environ Health Perspect 108 (suppl 1): 101-112.
- Villa-Guillen DE (2020) Breast cancer and exposure to hazardous contaminants: A minefield of feats and challenges. Open Acc J Bio Sci 2(1): 303-305.
- INEGI (National Institute of Statistics and Geography) (2015) Defunciones de mujeres por tumor maligno de la mama por entidad federativa y grupo quinquenal de edad, 2010 a 2015. INEGI, Mexico.
- Duarte-Tagles HF (2003) CYTRAR Detrás de los residuos peligrosos en Sonora. Universidad de Guanajuato. Guanajuato, México. Acta Universitaria 13(2): 14-21.
- CCA (Comisión de Cooperación Ambiental de América del Norte) (2010) México Quinta Comunicación Nacional ante la Convención Marco de las Naciones Unidas sobre el Cambio Climático. Portal de América del Norte sobre contaminantes precursores del cambio climático.
- Sánchez-Osorio JL (2017) Organochlorine pesticides in residential soils and sediments within two main agricultural areas of northwest Mexico: Concentrations, enantiomer compositions and potential sources. Chemosphere 173: 275-287.
- SINA (Sistema Nacional de Información de Salud) (2011) Estadísticas del cáncer de mama. SINAIS, México.
- CONAGUA (Comisión Nacional del Agua) (2012) Estadísticas del Agua en México edición 2012. Comisión Nacional del Agua, SEMARNAT, México.
- Castrezana-Campos MR (2017) The geography of Mexico breast cancer. Investigaciones Geográficas UNAM ISSN (digital).
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2): 377-381.
- LFM (Leyes Federales de México) (2010) Ley Federal de Protección de Datos Personales en Posesión de los Particulares.
- (2012) DOF (Diario Oficial de la Federación) NORMA Oficial Mexicana NOM-024-SSA3-2012, Sistemas de información de registro electrónico para la salud.
- (2015) HHS (US) Department of health and human services, office of the secretary, office for civil rights & Ocr). Methods for De-identification of PHI.
- Brender JD, Maantay JA, Chakraborty J (2011) Residential proximity to environmental hazards and adverse health outcomes. AJPH 101: S37-S52.
- SSA (Secretaría de Salud) (2003) Reglamento de la ley general de salud en materia de prestación de servicios de atención médica.
- NIHS (National Center for Health Statistics) (2017) Adult tobacco use - Glossary.
- CDC (Centers for Disease Control and Prevention) (2020) Alcohol and public health.
- Huang PL (2009) A comprehensive definition for metabolic syndrome. Dis Model Mech 2(5-6): 231- 237.
- INEGI (Instituto Nacional de Estadistica y Geografia) (2016) Censo de Poblacion y Vivienda 2010.
- Arthur R, Wassertheil-Smoller S, Manson JE, Luo J, Snetselaar L (2018) The combined association of modifiable risk factors with breast cancer risk in the women’s health initiative. Cancer Prev Res 11(6): 317-326.
- Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T (2010) Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res 12: 207.
- Saadatmand S, Bretveld R, Siesling S (2015) Influence of tumour stage at breast cancer detection on survival in modern times: population-based study in 173 797 patients. BMJ 2015: 351.
- Rowell MD, Perry RR, Hsiu JG, Barranco SC (1993) Phyllodes tumors. Am J Surg 165 (3): 376-379.
- Tu H, Wen CP, Tsai SP, Chow WH, Wen C (2018) Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ (Clinical research ed.) 360: k134.
- Wang AT, Vachon CM, Brandt KR, Ghosh K (2014) Breast Density and Breast Cancer Risk: A Practical Review. Mayo Clin Proc 9(4): 548- 557.
- Yaghjyan L, Tamimi RM, Bertrand KA, Scott CG, Jensen MR (2017) Interaction of mammographic breast density with menopausal status and postmenopausal hormone use in relation to the risk of aggressive breast cancer subtypes. Breast Cancer Res Treat 165(2): 421-431.
- Duffy SW, Morrish OWE, Allgood PC, Black R, Gillan MGC (2018) Mammographic density and breast cancer risk in breast screening assessment cases and women with a family history of breast cancer. Eur J Cancer 88: 48-56.
- Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE (2019) The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep 21(5): 41.
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM (2017) Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 67 (5): 378-397.
- Lahart IM, Metsios GS, Nevill AM, Carmichael AR (2015) Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol 2015 54: 635-654.
- Wani B, Aziz SA, Ganaie MA, Mir MH (2017) Metabolic syndrome and breast cancer risk. Indian journal of medical and paediatric oncology. Indian J Med Paediatr Oncol 38(4): 434-439.
- Li P, Wang T, Zeng C, Yang M, Li G (2020) Association between metabolic syndrome and prognosis of breast cancer: a meta-analysis of follow-up studies. Diabetol Metab Syndr 12: 10.
- Lo JJ, Park Y-MM, Sinha R, Dale P Sandler DP (2020) Association between meat consumption and risk of breast cancer: Findings from the Sister Study. Int J Cancer 146(8): 2156-2165.
- Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S (2017) Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 377: 2228-2239.
- CSA (Catholic Straight Answers) (2013) What is the church’s teaching on contraception?
- Knight JA, Fan J, Malone KE, John EM, Lynch CF (2017) Alcohol consumption and cigarette smoking in combination: A predictor of contralateral breast cancer risk in the WECARE study. Int J Cancer 141(5): 916-924.
- Adami HO, Lund E, Bergström R, Meirik O (1988) Cigarette smoking, alcohol consumption and risk of breast cancer in young women. British Journal of Cancer 58(6): 832-837.
- Kana MA, Ari M, Solomon P, Lunet N (2015) Arquivos de medicina 29 (5): 132-134.
- Fortner RT, Sisti J, Chai B, Collins LC, Rosner B (2019) Parity, breastfeeding and breast cancer risk by hormone receptor status and molecular phenotype: results from the nurses’ health studies. Breast Cancer Res 21: 40.
- Redondo CM, Gago-Dominguez M, Miranda-Ponte S, Enguix-Castelo M, Jiang X (2012) Breast feeding, parity and breast cancer subtypes in a Spanish cohort. PLOS ONE 7(7): e40543.
- Liu Y, Zhang J, Huang R, Feng WL, Kong YN (2017) Influence of occupation and education level on breast cancer stage at diagnosis, and treatment options in China: A nationwide, multicenter 10-year epidemiological study. Medicine (Baltimore) 96(15): e6641.
Article Type
Research Article
Publication history
Received date: June 25, 2020
Published date: July 16, 2020
Address for correspondence
Diana Evelyn Villa-Guillen, Ph.D. Universidad de Sonora, Blvd. Luis Encinas J, Centro, Mexico
Copyright
©2020 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
DE Villa-Guillen, E Avila-Monteverde, JH Gonzalez-Zepeda, LF Munguia-Ibarra, B Corral-Villegas, KG Aguilar-Gutierrez, C Rojas-Camarena, LC Durazo- Cons, CD Luque-Morales, WB Aguilar-Peraza, JA Cordon-Guillen, E Ruiz-Bustos, Jorge Villa Carrillo. Breast Cancer Prognosis and Exposure to Hazardous Contaminants: An Observational Retrospective Study at Hermosillo, Sonora, Mexico. 2020 - 2(4) OAJBS.ID.000192.
Figure 1: Conditional independence of breast cancer patients residentially exposed and their age given their 5-year prognosis for survival. Abbreviations: Exposed, residential exposure to hazards; Age, age at the time of breast cancer diagnosis; Prognosis, 5-year breast cancer prognosis. Model 1: (Exposed + Age) * Prognosis. p-value = 0.004.
Figure 2: Conditional independence of breast cancer patients residentially exposed and their serum blood cholesterol given their 5-year prognosis for survival. Abbreviations: Exposed, residential exposure to hazards; Cholesterol, high fasting serum blood cholesterol (> 200 mg/dl) at the time of diagnosis; Prognosis, 5-year breast cancer prognosis. Model 2: (Exposed + Cholesterol) * Prognosis. p-value = 0.021.
Figure 3: Conditional independence of breast cancer patients residentially exposed and their serum blood triglycerides given their 5-year prognosis for survival. Abbreviations: Exposed, residential exposure to hazards (either GPP, RWS, or HAP-exposed); Triglycerides, high fasting serum blood triglycerides (> 150 mg/dl) at the time of diagnosis; Prognosis, 5-year breast cancer prognosis. Model 3: (Exposed + Triglycerides) * Prognosis. p-value = 0.043.
Figure 4: Joint independence of 5-year prognosis and the combination of hazard exposure and previous cancer(s) in breast cancer patients. Abbreviations: Exposed, residential exposure to hazards; Previous Cancer, cancer(s) before the time of diagnosis; Prognosis, 5-year breast cancer prognosis. Model 4: Exposed * Previous Cancer + Prognosis. p-value = 0.027.
Figure 5: Complete independence of 5-year prognosis, hazard exposure, and diabetes mellitus type 2 in breast cancer patients. Abbreviations: Exposed, residential exposure to hazards; Diabetes, diabetes mellitus type 2 before the time of diagnosis; Prognosis, 5-year breast cancer prognosis. Model 5: Exposed + Diabetes + Prognosis. p-value = 0.034.
Figure 6: Complete independence of 5-year prognosis, hazard exposure, and menopausal status in breast cancer patients. Abbreviations: Exposed, residential exposure to hazards; Menopausal status, the menopausal status at the time of diagnosis (labeled as 0 for premenopausal, 1 for perimenopausal, and 2 for postmenopausal); Prognosis, 5-year breast cancer prognosis. Model 6: Exposed + Menopausal Status + Prognosis. p-value = 0.048.
Figure 7: Complete independence of 5-year prognosis, hazard exposure, age, and previous cancer(s) in breast cancer patients. Abbreviations: Exposed, residential exposure to hazards; Age, the age at the time of diagnosis; Previous Cancer, cancer(s) diagnosed before current disease; Prognosis, 5-year breast cancer prognosis. Model 7: Exposed + Age + Previous Cancer + Prognosis. p-value = 0. 001.
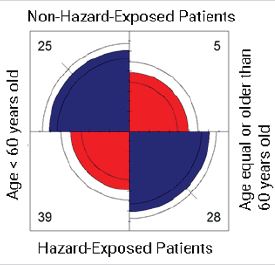
Figure S1: Comparison of the breast cancer patients with an overall bad 5-year prognosis and residentially exposed to hazards. Age groups are divided into two categories: patients < 60 years old, and patients ≥ 60 years old. Patients residentially exposed to hazards were those reporting to living in a highly industrialized neighborhood (> 6 industrial facilities) considered GPP-exposed (within a radial distance of 4 km) and RWS-exposed (within a radial distance of 3 km). OR = 3.59 (1.22, 10.52), p-value = 0.02. The likelihood is higher for hazard-exposed patients with an age equal or older than 60 years old (28), and for non-hazardexposed patients younger than 60 years old (25).
Figure S2: Comparison of the breast cancer patients with an overall intermediate 5-year prognosis and residentially exposed to hazards. Age groups are divided into two categories: patients < 60 years old, and patients ≥ 60 years old. Patients residentially exposed to hazards were those reporting to living in a highly industrialized neighborhood (> 6 industrial facilities) considered GPP-exposed (within a radial distance of 4 km) and RWS-exposed (within a radial distance of 3 km). OR = 2.68 (0.99, 7.24), p-value = 0.05. The likelihood is higher for hazard-exposed patients with an age equal or older than 60 years old (30), and for non-hazard-exposed patients younger than 60 years old (20).
Figure S3: Comparison of the breast cancer patients with an overall good 5-year prognosis and residentially exposed to hazards. Age groups are divided into two categories: patients < 60 years old, and patients ≥ 60 years old. Patients residentially exposed to hazards were those reporting to living in a highly industrialized neighborhood (> 6 industrial facilities) considered GPP-exposed (within a radial distance of 4 km) and RWS-exposed (within a radial distance of 3 km). OR = 2.89, (0.99, 8.35), p-value = 0.05. The likelihood is higher for hazard-exposed patients with an age equal or older than 60 years old (23), and for non-hazardexposed patients younger than 60 years old (32).
Table 1: Models which include breast cancer risk factors, residential exposure to hazards, and 5-year breast cancer prognosis where the null hypothesis is rejected.
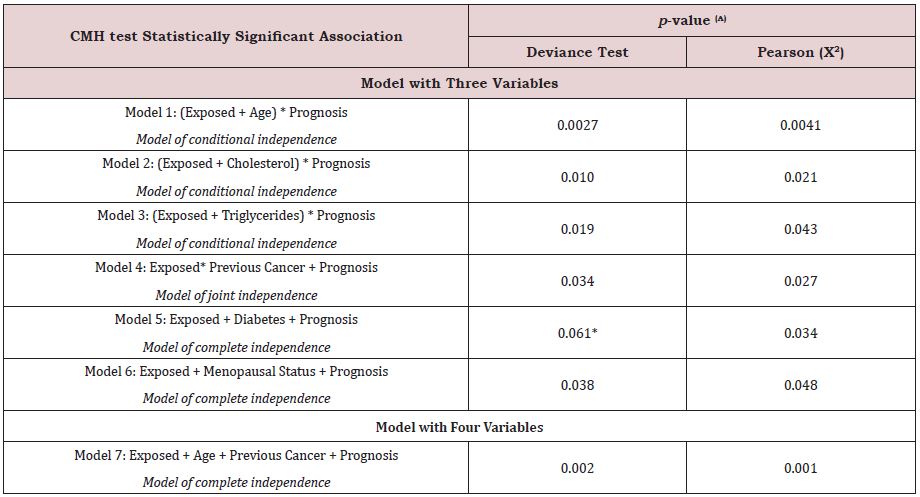
Table 1 Abbreviations: Exposed, residential exposure to contaminants (GPP, RWS, HAPs); Age, age at the time of breast
cancer diagnosis; Prognosis, 5-year breast cancer prognosis; Cholesterol, serum fasting blood cholesterol > 200 mg/dl;
Triglycerides, serum fasting blood triglycerides > 150 mg/dl; Previous Cancer, any cancer(s) diagnosed prior the current
breast cancer; Diabetes, diabetes mellitus type 2; Menopausal Status, the menopausal status of the cohort classified onto
three categories (premenopausal, perimenopausal, postmenopausal).
(A) Statistical significance if p-value ≤ 0.05 for all models, except (*). This last model fitted for deviance test, but not for
Pearson (X2), considering a p-value = 0.05. R version 3.4.2.
Table 2: Models for potential breast cancer risk factors, residential exposure to hazards, and 5-year breast cancer prognosis where the null hypothesis is accepted.
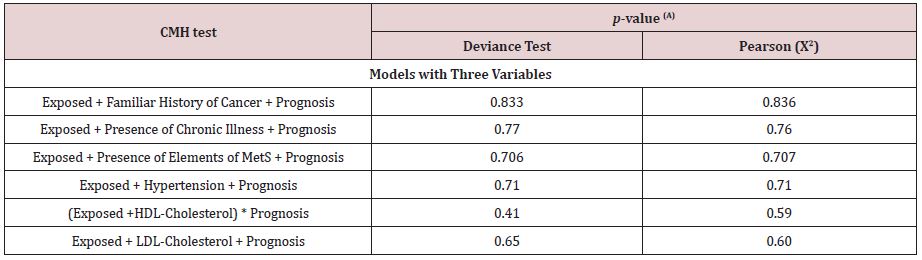
Table 2 Abbreviations: Exposed, residentially exposed to hazards (GPP, RWS, HAP); Prognosis, 5-year breast cancer
prognosis of the cohort, Familiar History of Cancer, includes any relative diagnosed with cancer; Presence of Chronic
Illness, includes any prior chronic disease before diagnosis; Presence of Elements of MetS, overall presence of elements of
metabolic syndrome as defined by the NCEP ATP III criteria [Huang]; Hypertension, blood pressure ≥ 130/80 mmHg; HDLCholesterol,
low HDL-cholesterol < 50 mg/dl (women); LDL-Cholesterol, high LDL-cholesterol ≥ 160 mg/dl.
(A) Statistical significance if p-value ≤ 0.05. R version 3.4.2.
Table S1a: Clinical characteristics of the study cohort (n=297) at Hermosillo, Sonora, Mexico. Variables include those from the time of breast cancer diagnosis, and at the status of the patient.
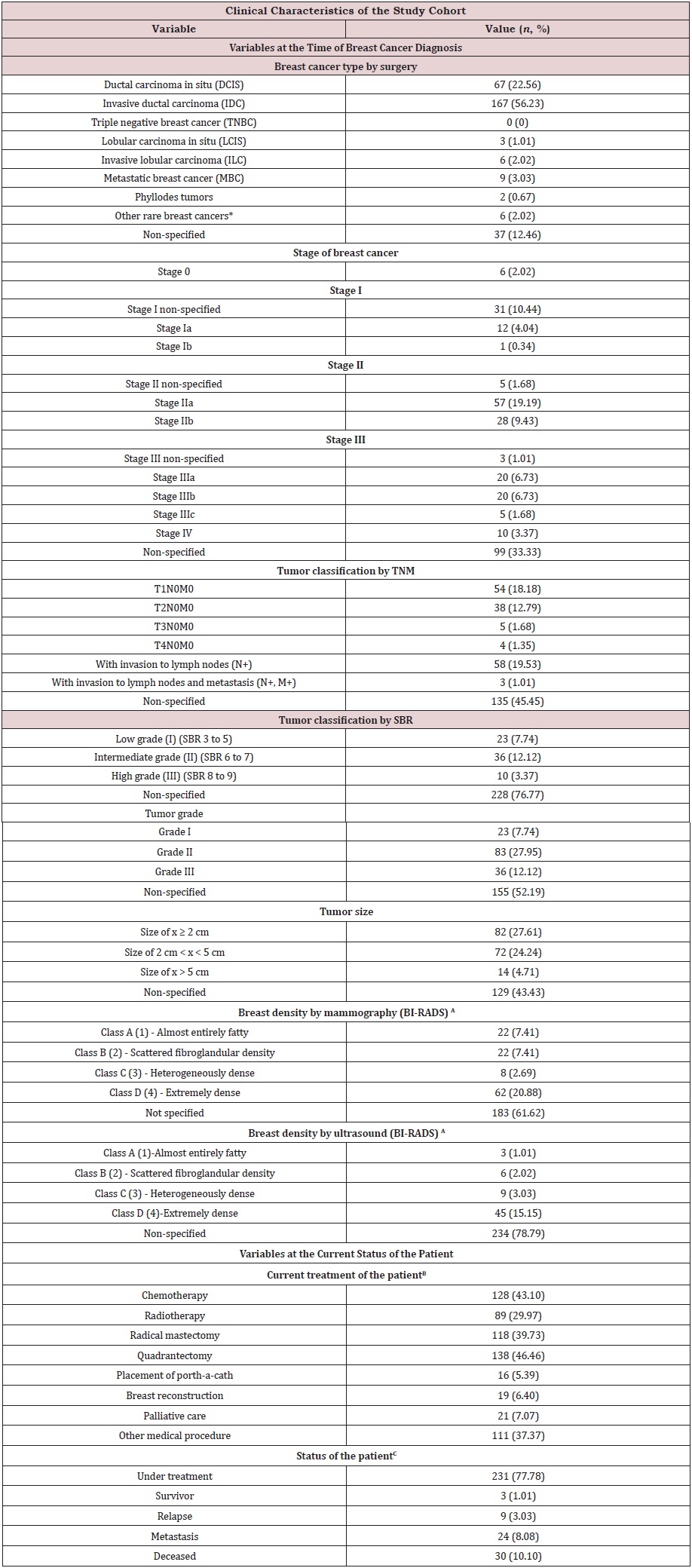
Table S1a Abbreviations: TNM: T-Tumor Size, N-Spread of Cancer to Nearby Lymph Nodes, M- Metastasis; SBR: Scarff-
Bloom-Richardson grading
(*) Other rare breast cancers: Medullary carcinoma of the breast (1); mucinous carcinoma of the breast (2); papillary
carcinoma of the breast (1); Inflammatory breast cancer (1); Bilateral breast cancer with subtype non-specified
(1).
(A) The breast density by mammography or ultrasound was that of at the time of breast cancer diagnosis. Clinical
database collected no other imaging reports after the patient’s diagnosis.
(B) Medical procedures conducted up to date of the present analysis. Patients initiating treatment at the time of data
collection had only placement of port-a-cath.
(C) Status of the patient according to the last medical record available on files.
Table S1b: Non-modifiable breast cancer risk factors of the study cohort (n=297) at Hermosillo, Sonora, Mexico. Race and ethnicity were not collected, as Hermosillo residents are Hispanics-only.
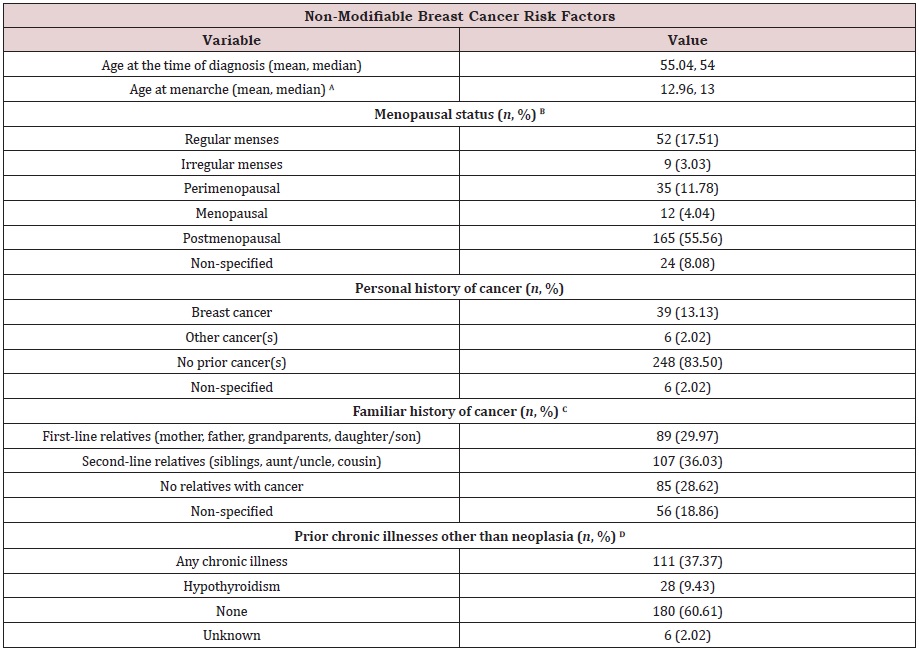
(A) Data regarding age at menarche was available for 69.70 % of files. 30.30 % of the cohort had no information.
(B) Menopausal status reported at the time of breast cancer diagnosis. Clinical database did not collect the current
menopausal status of the patient.
(C) Familiar history as reported on files. Some patients reported more than one relative diagnosed with cancer.
(D) Chronic illnesses reported on files at the time of breast cancer diagnosis. Clinical database did not collect chronic
diseases reported after the diagnosis. Other chronic illnesses besides hypothyroidism were dyslipidemia (4), vesicular lithiasis
(1), alcoholism (1), schizophrenia (2), uterine myomatosis (7), thyroidectomy given prior thyroid cancer or hyperthyroidism
(1), cervicitis (1), carpal syndrome (1), hyperthyroidism (1), endometrial hyperplasia (2), chronic venous insufficiency (5),
convulsive crisis given mental illnesses (3), rheumatoid arthritis (5), anemia (3), hepatic steatosis (5), cholecystectomy
given suspicious malignancies (4), diabetes mellitus type 2 (19), hypertension (37), Guillain-Barre syndrome (1), morbid
obesity (5), hypotension (3), attention-deficit/hyperactivity disorder (1), hypercholesterolemia (3), pancreatitis (2), ovarian
cysts (2), nephritis (1), chronic cholecystitis (1), gonarthrosis (1), fibrosis colonopathy (1), osteoporosis (1), mumps (1),
adenomegaly (1), ischemic cardiomyopathy (1), partial blindness (2), brain paralysis (1), brain aneurysm (1), asthma (2),
chronic gastritis (1). Some patients reported more than one chronic illness prior breast cancer diagnosis.
Table S1c: Modifiable breast cancer risk factors of the study cohort (n=297) at Hermosillo, Sonora, Mexico.
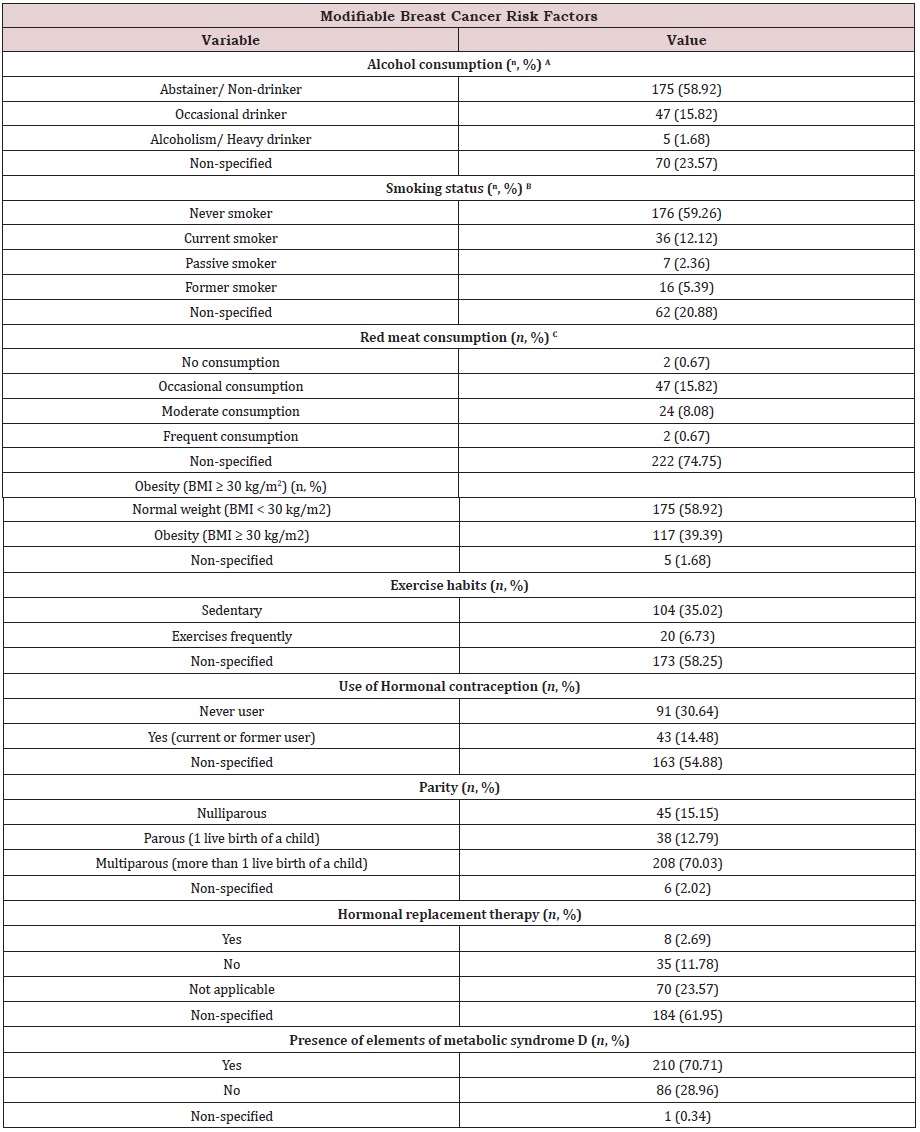
(A) Alcohol consumption was defined according to the Centers for Disease Control and Prevention (CDC) guidelines,
US CDC [39]. In women, heavy drinking is typically defined as consuming 8 cups of alcoholic beverages or more per week.
Occasional drinking is considered 7 cups or less per week. Abstainer or non-drinker is defined as a woman with nonalcohol
consumption, or that of 2 times or less per year.
(B) Smoking status was defined according to the National Health Interview Survey (NHIS), US NHIS [38]. Definitions for
each category: Never smoker, an adult who has never smoked, or who has smoked less than 100 cigarettes in her/his
lifetime; Current smoker, an adult who has smoked 100 cigarettes in her/his lifetime and who currently smokes cigarettes;
Passive smoker, also called second-hand smoke, usually refers to cigarette smoke in the environment of a nonsmoker;
Former smoker, an adult who has smoked 100 cigarettes in her/his lifetime but who had quit smoking at the time of the
interview. Absence of clinical data classified as non-specified.
(C) Red meat consumption was defined according to nutritional habits per week. Non-consumption includes
veganism, or white meat consumption, with no red meat consumption per week. Occasional consumption entails
red meat consumption 1-2 times per week. Moderate consumption entails red meat consumption 3-4 times per week.
Frequent consumption entails red meat consumption 5-7 times per week.
(D) The elements of metabolic syndrome were defined according to the NCEP ATP III criteria Huang [40]. Those are
waist circumference over 35 inches (women), blood pressure over 130/85 mmHg or in medical treatment for it, fasting
triglycerides > 150 mg/dl, fasting high-density lipoprotein (HDL) cholesterol < 50 mg/dl (women), and fasting glucose > 100
mg/dl.
Table S1d: Education, religion, and occupation of the study cohort (n=297) at Hermosillo, Sonora, Mexico.
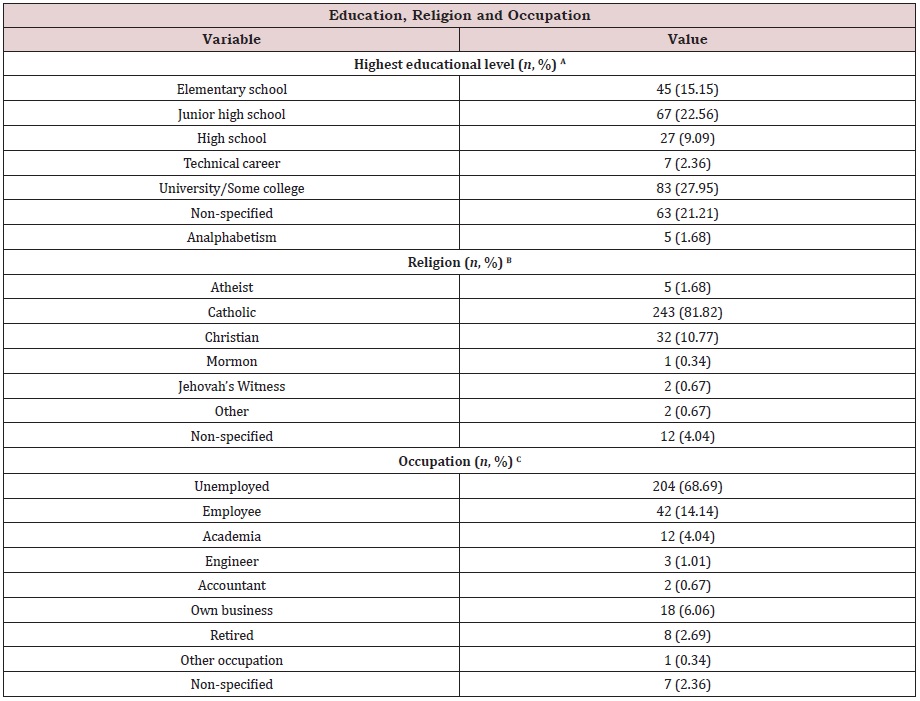
(A) The highest educational level achieved by the patient was that of reported by herself on clinical files. Highest
level included its enrollment-only, but not its completion.
(B) The religion expressed (active or inactive) by the patient as reported on her clinical files.
(C) Current occupation of the patient at the time of diagnosis. Unemployed indicates no current job position and
reports as a housewife, employee comprises any other job than engineer, accountant, academia, or own business. Retired
includes pensioned. Other occupation includes informal jobs or by contract. Non-specified indicates no information
available on files.