A Survey of Iranian Population Attitude About Vaccination Against COVID-19 and Evaluation of Its Spontaneous Reporting of Adverse Effects
ABSTRACT
The COVID-19 pandemic continues to claim victims around the world, and Iran is no exception from this ravage. Vaccine
development led to growing optimism regarding control of COVID-19. Vaccine acceptance by the majority of the population
is important for the success in controlling COVID-19. This study assessed the attitude of Iranian population toward COVID-19
vaccination. Also, the adverse effects of the COVID-19 vaccines on vaccinated individuals were reported. This cross-sectional
study was conducted from May to July 2021 among Iranian population. The data was collected using the online researcher-made
questionnaire. The questionnaire contained 16 questions in 3 sections.
The questionnaire was filled out by 916 people in total. The majority of participants had 40-65 years old (N:480, 52.4%). More
than half of participants were from health care team (N:543, 59.3%). The majority of participants trusted the vaccine and said
they would inject it (N:582, 63.5%). However more than 80% of participants preferred non-domestic vaccines over Iranian ones.
Potential adverse effect following vaccination was the main concern of responders (N:576, 62.9%). The most common adverse
effects reported by vaccine recipients were temporary fatigue and muscle pain (N:313, 71.95%). The level of education, academic
area and area of practice in the health system were significantly associated with the positive response to COVID-19 vaccination
(P-value 0.0001, 0.0001 and 0.004 respectively). According to the results of this study, Iranian health authorities should provide
clear information about the safety and efficacy of COVID-19 vaccines, especially domestic types, to increase public confidence and
awareness regarding vaccines.
KEYWORDS
COVID-19; Vaccination; Iranian population; Attitude; Adverse effect
INTRODUCTION
Since the first cases of COVID-19, were identified more than a year ago. Approximately 220 countries were affected by the COVID-19 outbreak starting in December 2019. The effects of COVID-19 vary from person to person. The majority of people who have COVID-19 infection will show mild to moderate symptoms and recover without hospitalization; however, some will undergo hospitalization or even die due to their illness [1]. More than 190 million cases and 4.09 million deaths worldwide have been attributed to the COVID-19 pandemic as of July 17,2021 [2]. Human health was not the only aspect of human life that was affected by this disease; economic conditions, and social relationships were also impacted [3]. It appears that the virus is transmitted mainly through the inhalation of droplets with a predominance of respiratory symptoms [4]. Several strategies were implemented to stop the spread of COVID-19, including social distancing, hand washing, face mask wearing, and reducing presence in crowded places [5]. Despite the fact that these methods have slowed disease spread, these methods are challenged by lack of public support or resilience, noncompliance with national and international guidelines, and inadequate infrastructure and resources [6].
As the disease had no specific treatment and many people died daily, the international community became focused on vaccination and increasing public immunity against it. Currently, a major public health measure that can save millions of lives worldwide is the vaccination. To date, different platforms including inactivated virus, recombinant protein, RNA based vaccine and deficient adenovirus based, have been used to manufacture vaccines [7]. As of 17 July 2021, human clinical trials have been conducted on 97 vaccines, and 32 vaccines have reached the final stages. Also, animal studies are ongoing on 77 preclinical vaccines [8]. As a result of the development of a vaccine against COVID-19, the human community gained hope for faster control of the disease. On the other hand, there are some concerns about safety, efficacy, and availability of COVID-19 vaccine among many people. Vaccine hesitancy remains a potential impediment to community uptake [9]. As defined by the WHO Strategic Advisory Group of Experts (SAGE), vaccine hesitancy is the refusal or delay of vaccination despite the availability of vaccination services [10]. The vaccine related adverse effects have a key role in public confidence in the vaccine. Fear of adverse effects is one of the reasons for people’s distrust and rejection of the vaccine. The information on COVID-19 vaccines adverse effects comes from vaccine manufacturers’ studies; so further observational studies can provide more information on vaccines adverse effects. Raising knowledge about different aspects of COVID-19 vaccines can help allay concerns and improve public confidence [11].
With a population of about 84 million, the COVID-19 infection rate in Iran is high. As of 17 July 2021, more than 3.5 million COVID-19 confirmed cases and over 86966 deaths have been reported in Iran2. Several vaccines from different companies have entered the clinical studies phase. Currently in Iran two domestically made vaccines have been approved for emergency use from the Ministry of Health of Iran, there are also a number of vaccines in various stages of development in different domestic institutions [12]. It is important to examine the attitudes regarding COVID-19 vaccines among Iranian population as well as their acceptance of the vaccines. This study was conducted to evaluate the attitude and acceptance of Iranian population toward COVID-19 vaccination. A study of the adverse effects of the COVID-19 vaccine on vaccinated individuals was also conducted, in light of the fact that the occurrence of adverse effects is one of the concerns of people about the vaccination. However, several studies have examined the tendency to receive vaccines, preference for domestic versus non-Iranian vaccines [13], priority groups for vaccination [14], and adverse effects of vaccine [15] among special groups in Iran. These issues, along with other factors affecting attitudes, are comprehensively addressed in this study.
MATERIALS AND METHODS
This survey is a cross-sectional study in which the attitude and acceptance of the Iranian population about COVID-19 vaccination were assessed. Vaccinated individuals were also evaluated for adverse effects of the vaccine. This study was conducted in August 2021. The study was reviewed and approved by the Ethics Committee of the Tehran University of medical sciences (IR.TUMS. TIPS.REC.1400.091). There was no consent form and completion of the questionnaire by each person was considered as her/ his satisfaction.
Data collection was conducted using the online researchermade questionnaire. The questionnaire contained 16 questions in 3 sections (Table 1-3). The first part consisted of 5 questions about demographic information including age, sex, level of education, and occupational field. In 6 questions of second part, attitude and acceptance of general population and healthcare staffs regarding COVID-19 vaccine were evaluated. The third section of the study focused on those who had been vaccinated against COVID-19 and asked the participants about the occurrence of adverse effect after vaccination, its duration, and measures taken to eliminate it. This questionnaire was designed and then tested for validity and reliability, and then converted into an online form. The questionnaire was uploaded to online social media platforms (WhatsApp, Telegram and Instagram). There are more than 59 million internet users in Iran [16]. We sent a questionnaire to various social groups, and members of the groups were asked to respond to the questions and share the questionnaire with their contacts or acquaintances. In order to prevent duplicate answers, the questionnaire was designed to be answered only once by each participant. The content validity of the Persian-language questionnaire was evaluated by 9 experts (physicians, pharmacists and university professors). Content validity index and the content validity ratio calculated 0.96 and 0.82 respectively.
All data were analyzed using Statistical Package for the Social Sciences (SPSS) version 25. Shapiro-Wilk’s test was utilized to assess the normality distributions of variables. Continuous variables were expressed as mean ± standard deviation or median [interquartile range]. Independent sample t-test and Mann– Whitney U-test were performed to compare normal and non normal quantitative variables between the two groups. Categorical variables were presented as numbers and percentages. Chi-square and Fisher exact test were used to compare categorical variables. In order to assess the association of acceptance of the participants and other variables, the answers “ I trust/ I inject” and “ I do not trust/ I inject “ were considered similar to yes and the options “ I do not trust/ I do not inject” and “I trust/ I do not inject “and “Due to catching COVID-19, I do not need a vaccine” were considered similar to no and were tested by Chi-square/ Fisher exact test. In all comparisons, P-values less than 0.05 were considered as significant.
The sample size was calculated with the Raosoft sample size calculator. In order to achieve a confidence level of 99% and margin of error of 5% for this study, a sample size of 664 people with a distribution of responses of 50% is required. A response rate of 30 to 40% is estimated for studies based on online questionnaires [17,18], so the questionnaire was uploaded in different social groups with a high number of members.
RESULTS
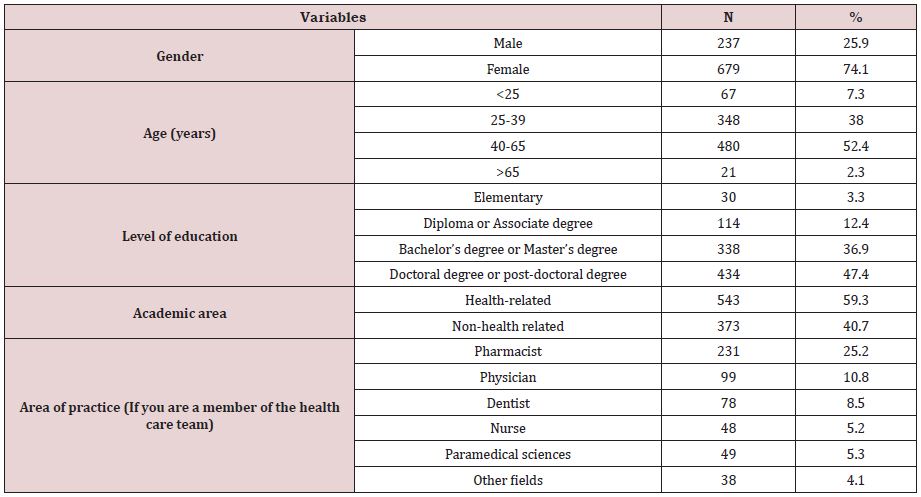
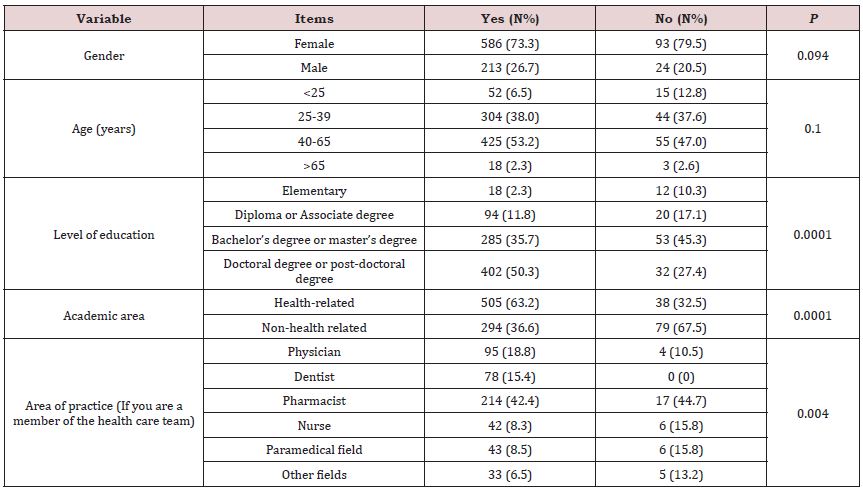
A total of 1836 people visited this questionnaire, but 916 participants completed it. The majority of participants had 40-65 years old (N:480, 52.4%) and more than 74% (N:679) of them were female. Most participants had high level of academic education (N: 434, 47.4%) doctoral or post-doctoral degree). More than half of participants were from health care team (N:543, 59.3%), whereas pharmacists constituted the majority (N:231, 25.2%). The sociodemographic characteristics of the participants were obtained in Table1.
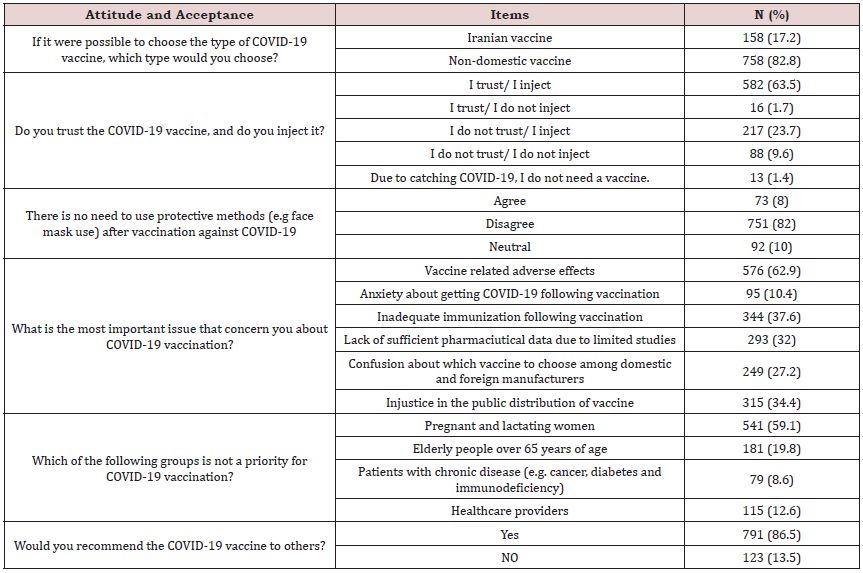
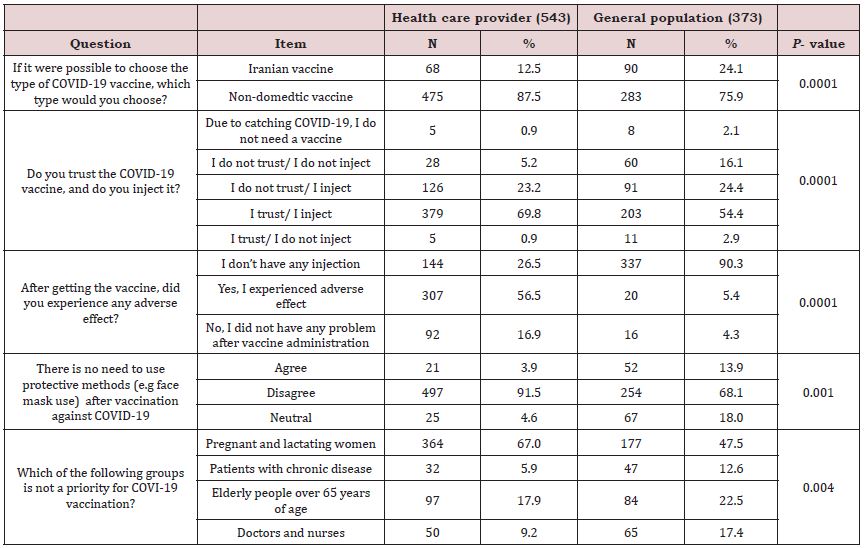
In terms of acceptance of COVID-19 vaccines, among respondents, more than 80% (N: 758) preferred non-domestic vaccines over Iranian ones. Nevertheless, the majority of participants trusted the vaccine and said they would inject it (N:582, 63.5%). Most participants, whether general population (N: 294, 78%) or health care team (N:505, 93%), tend to injected vaccine; but the percentage of people who refuse to inject vaccine was significantly higher for the general population (N:79, 19%) than for medical staff (N:38, 6.1%). Besides, 86.5% (N: 791) reported they recommend COVID-19 vaccine administration to others. Potential adverse effects following vaccination (N:576, 62.9%), as well as the possibility of inadequate immunization (N:344, 37.6%), were the main concerns of participants regarding COVID-19 vaccination. Most participants were well-informed about COVID-19 vaccination; they knew group priorities for vaccination (N:541, 59.1%) and believed that vaccination would not eliminate the need for protective measures (e.g., face masks) (N:751, 82 %). Compared to the general population, medical staff had more correct answers to questions dealing with vaccination priorities (67% vs 47.5%, P-value: 0.004) and need for protective measures after vaccination (91.5% vs 68.1%, P-value: 0.001). The comparison between health care providers and the general population is shown in Table 4.
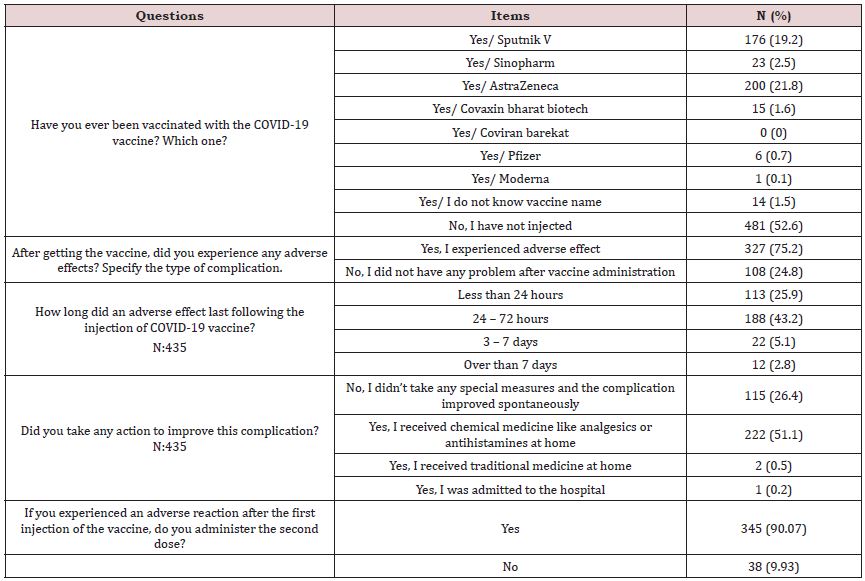
In this study, almost half of participants (435 participants, 47.4%) received COVID-19 vaccine, AstraZeneca® vaccine was mostly given to them (N: 200, 21.8%). Approximately 75.2 percent of participants (N:327) experienced one or more adverse effects after receiving the vaccine. The most common adverse effects reported by vaccine recipients were fatigue and muscle pain (N:313, 71.95%). Most vaccine adverse effects usually resolved by analgesics or antihistamines (N: 222, 51.1%) within 24 to 72 hours (N: 188, 43.2%), and only 2.8% (N:12) of cases lasted longer than a week. Majority of respondents (more than 90%, N:345) who had received the first dose of the vaccine expressed a desire to receive the next dose.
As shown in Table 5, level of education, academic area and area of practice in the health system were significantly associated with the positive response to COVID-19 vaccination (P-value 0.0001, 0.0001 and 0.004 respectively).
DISCUSSION
This study was conducted to investigate the acceptance and attitude of Iranian population toward COVID-19 vaccines. Additionally, adverse reactions following the vaccination and how to control them were investigated in vaccinated individuals.
Many people around the world are concerned about the use of the COVID-19 vaccine because of the lack of global experience with it, doubts about its effectiveness and potential adverse effects. Among the 916 participants in this study, 87.2% were eager to receive the vaccine. Surprisingly, 23.7% of respondents (N: 217) said that they still inject vaccines despite not trusting them. There have been a variety of studies conducted in different countries to determine the acceptability of the COVID-19 vaccine. In a global survey, the COVID-19 acceptance of 13426 people from 19 countries was assessed. The highest acceptance rates were found in China (88.62%), followed by Brazil (85.36%), and the lowest rates were found in the Russian population (54.85%) [19]. There were acceptance rates of 53.1% in Kuwait [20], 37.4% in Jordan [4], and 64.7% in Saudi Arabia [21] among Asian countries. The present study found that health care providers accept the COVID-19 vaccine more than the general population. It can be attributed to the higher level of knowledge in the field of COVID-19, possibility of further COVID-19 infection because of special occupational conditions among health care providers, as well as the direct observation of morbidity and mortality of this disease. In the study of Fares et al, in line with our results, it was observed that factors such as direct contact with patients with COVID-19 and obtaining sufficient information about COVID-19 vaccine have a significant effect on increasing the acceptance of health care workers [22].
This study found that people may be concerned about vaccinations for a variety of reasons. Participants most commonly cited possible complications following vaccinations, poor vaccination efficacy, and the lack of enough scientific backing to make the vaccine. These results are in line with those reported by Pogue and colleagues. They evaluated the attitude of Americans toward their vaccination against COVID-19. According to their study, most people were concerned by the adverse effects of vaccines, uncertain efficacy, and the short duration of vaccine studies [23]. In El-Elimat ‘s study, vaccine safety concerns were cited as a significant reason for vaccine rejection in the Jordanian population [4]. The present study showed that a large percentage of participants were willing to receive the vaccine, but that most prefer the non-Iranian vaccine to the Iranian vaccine. Similarly, 73% of Iranian professors and researchers who participated in another study conducted in August 2020 reported their preference for non-Iranian vaccines [13]. The distrust may have been caused, in part, by the idea that domestic products are inferior to foreign counterparts, as vaccines did as well as many other domestic items. In addition, the misinformation and worrying rumors about domestic vaccines sparked public concern. Increased transparency by vaccine manufacturers and government officials regarding the production and distribution of locally produced vaccines can lead to greater public confidence in the field of domestic COVID-19 vaccines.
There is a need to prioritize the population to receive the vaccine in Iran, in light of the large population and the lack of adequate access to vaccine for all members at the same time; those involved in the study were asked to select the priority groups. The results showed that patients with chronic disease, healthcare providers and elderly population were reported as prioritized groups for COVID-19 vaccination. These groups were currently prioritized for vaccination by Iranian health policymakers. The results were similar to those found by Moradi et al., who surveyed 878 Iranians about priority groups for vaccination [14]. This study asked the participants about wearing masks and attention to protective methods after receiving the vaccine. Most participants, especially healthcare providers, believed that it was necessary to wear a mask following vaccination against COVID-19. The center for disease control and prevention (CDC) now recommends that persons who have been vaccinated use masks to protect themselves, particularly against the new variant of COVID-19, and to avoid spread to others [24].
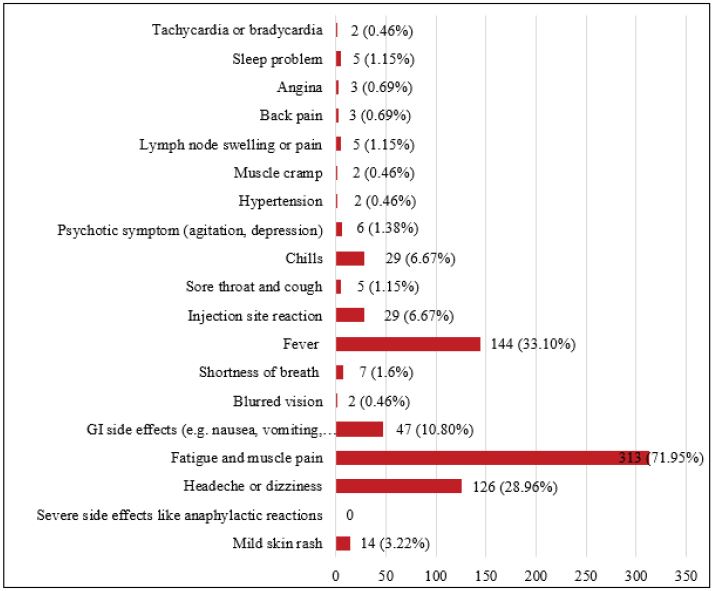
In another part of the study, people who had received the COVID-19 vaccine were asked about the occurrence of adverse effects following vaccination and how to manage it. During the study period, more healthcare providers were vaccinated than other populations. Participants received the AstraZeneca® vaccine at a higher proportion than other vaccines. This was due to greater imports of AstraZeneca® vaccine than other brands at the time of this study. Approximately 75% of participants experienced adverse effects after receiving the COVID-19 vaccine, with fatigue and muscle pain, fever, and headache being the most common complaints, respectively. The reported adverse effects were similar to those reported by the CDC, which identified tiredness, headache, muscle pain and fever as common adverse effects [25]. Fortunately, participants did not report life-threatening complications or anaphylaxis. A few participants also reported uncommon but important symptoms such as psychotic symptoms (e.g., restlessness and mood fluctuations), angina, and arrhythmia although they were temporary (Figure 1). At this time, these complications are rare and only reported in some case reports [26,27]. Most participants reported improvement in adverse effects after taking analgesics or antipyretics for 24 to 72 hours. The number of cases that required hospitalization from vaccination adverse effects was very low in this study.
This study was performed before vaccine was administered to a high percentage of the Iranian population. Therefore, people’s perceptions about the vaccine or the pattern of adverse effects coming from the vaccine may change as the vaccine is distributed further. Additionally, the study didn’t include all groups of people because those without access to mobile phones, computers, and the internet were unable to participate.
CONCLUSION
In conclusion, this study assessed the attitude of Iranian population toward COVID-19 vaccination. Also, the adverse effects of the COVID-19 vaccine on vaccinated individuals were evaluated. The majority of participants trusted the vaccine and said they would inject it. However more than 80% of participants preferred non-domestic vaccines. Although participants were worried about receiving the vaccine due to fears of negative adverse effects, fatigue and muscle pain were the most common adverse effects reported by participants. No life-threatening complications were reported as well. However, more studies need to be done with more participants in order to evaluate the adverse effects of COVID-19 vaccines.
REFERENCES
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, et al. (2021) Post-acute COVID-19 syndrome. Nat Med 27(4): 601-615.
- (2021) Reported cases and deaths by country or territory.
- Haleem A, Javaid M, Vaishya R (2020) Effects of COVID-19 pandemic in daily life. CMRP 10(2): 78-79.
- El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ (2021) Acceptance and attitudes toward COVID-19 vaccines: A crosssectional study from Jordan. PLoS one16(4): e0250555.
- (2021) How to Protect Yourself & Others, Content source: National Center for Immunization and Respiratory Diseases.
- Maqbool A, Khan NZ (2020) Analyzing barriers for implementation of public health and social measures to prevent the transmission of COVID-19 disease using DEMATEL method. Diabetes Metab Syndr14(5): 887-892.
- Nagy A, Alhatlani B (2021) An overview of current COVID-19 vaccine platforms. Comput Struct Biotechnol J 19: 2508-2517.
- Zimmer C, Corum J, Wee SL (2021) Coronavirus vaccine tracker US: The New York Times.
- Cordina M, Lauri MA, Lauri J (2021) Attitudes towards COVID-19 vaccination, vaccine hesitancy and intention to take the vaccine. Pharmacy practice 19(1): 2317-2317.
- Butler R, MacDonald NE (2015) Diagnosing the determinants of vaccine hesitancy in specific subgroups: The Guide to Tailoring Immunization Programmes (TIP). Vaccine 33(34): 4176-4179.
- Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, et al. (2021) Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med 10(7): 1428.
- Islamic republic of Iran ministry of health and medical education.
- Abouee-Mehrizi A (2021) A national survey of Iranian academicians’ attitudes towards covid-19 vaccination. CJHR 6(1): 21-28.
- Moradi N, Heydari S, Zarei Z, Arabloo J, Rezapour A, et al. (2021) Public views on priority groups for covid-19 vaccination: a survey from Iran. SEMJ 22(7): e113359.
- Babamahmoodi F, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Mousavi A, et al. (2021) Side effects and immunogenicity following administration of the sputnik v covid-19 vaccine among health care workers; an observational study in Iran. Preprint.
- Digital 2021: Iran.
- Fincham JE (2008) Response rates and responsiveness for surveys, standards and the journal. Am J Pharm Educ 72(2): 43-43.
- Regmi PR, Waithaka E, Paudyal A, Simkhada P, van Teijlingen E (2016) Guide to the design and application of online questionnaire surveys. Nepal J Epidemiol 6(4): 640-644.
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, et al. (2021) A global survey of potential acceptance of a COVID-19 vaccine. Nat med 27(2): 225-228.
- Alqudeimat Y, Alenezi D, AlHajri B, Alfouzan H, Almokhaizeem Z, et al. (2021) Acceptance of a COVID-19 vaccine and its related determinants among the general adult population in Kuwait. Med Princ Pract 30(3): 262-271.
- Al-Mohaithef M, Padhi BK (2020) Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J Multidiscip Healthc 13: 1657-1663.
- Fares S, Elmnyer MM, Mohamed SS, Elsayed R (2021) COVID-19 vaccination perception and attitude among healthcare workers in Egypt. J Prim Care Community Health 12.
- Pogue K, Jensen JL, Stancil CK, Ferguson DG, Hughes SJ, et al. (2020) Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines 8(4): 582.
- When You’ve Been Fully Vaccinated (2021) Center for disease control and prevention.
- Possible side effects after getting a COVID-19 vaccine (2021) Center for disease control and prevention.
- Jeet Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, et al. (2021) Cardiovascular adverse events reported from COVID-19 vaccines: a study based on WHO database. Int J Gen Med 14: 3909-3927.
- Srinivasan KN, Sathyamurthy I, Neelagandan M (2021) Relation between COVID-19 vaccination and myocardial infarction -Casual or coincidental? IHJ CVCR 5(2): 71-74.
Article Type
Research Article
Publication history
Received date: October 08, 2021
Published date: October 20, 2021
Address for correspondence
Elliyeh Ghadrdan, Assistant Professor, Department of Clinical Pharmacy, Faculty of Pharmacy, Alborz University of Medical Sciences, Iran
Copyright
©2021 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Sevda Micaeili-M, Soha N, Elliyeh G. A Survey of Iranian Population Attitude About Vaccination Against COVID-19 and Evaluation of Its Spontaneous Reporting of Adverse Effects. 2021- 3(5) OAJBS.ID.000337.
Figure 1: Incidence of COVID-19 vaccines related adverse effects.
Table 1: The demographic characteristics of the study participants (N=916).
Table 2: Attitude and acceptance toward COVID-19 vaccination.
Table 3: Evaluate adverse effects following COVID-19 vaccine administration.
Table 4: COVID-19 vaccination acceptance and adverse effects among healthcare providers and general population in Iran.
Table 5: Participant’s characteristics and association with COVID-19 vaccination acceptance.