Two-Sided RdRp SARS-CoV-2 and SARS-CoV-2 Mpro Selective Targeting Approach for COVID-19 Pandemic Therapy
ABSTRACT
Since the emergence of coronavirus disease (COVID-19) pandemic induced by SARS-CoV-2, the world is being suffered from a serious threat to public health through several hospitalizations and thousands of deaths leading to boosting global concern and intensive precautionary measurements. Exploring the key targets of SARS-CoV-2 for the evolution of efficient therapeutics has become dire of need. Our viewpoint provides current therapeutic targets and insights for advanced COVID-19 therapies to date and discusses new structural design of a potential therapeutic drug (Coronavir SJ) posing an innovative modality and framework for COVID-19 therapy, which, in turn, mediates different pattern for curbing greater SARS-CoV-2 virulence. Coronavir SJ is a prodrug providing two bioactive metabolites once administered in the blood for targeting definite RdRp SARS-CoV-2 and SARS-CoV-2 Mpro binding pockets, thereby displaying versatile curative functions. Although this design might be evidenced insufficient, we hypothesise that this therapeutic approach can open up pharmacological direction and guided avenues for basic experiments and research on COVID-19 infection and superspreading at this moment.
INTRODUCTION
SARS-CoV-2 was first identified in December 2019, belongs to Coronaviridae family, which encompasses a massive variety of regularly widespread viruses in animals and humans. The major manifestations in humans are mild respiratory or digestive disturbances [1]. Otherwise, minor coronaviruses, for instance, MERS-CoV, SARS-CoV-1, and SARS-CoV-2 have dense and serious respiratory disorders leading to lethal outbreaks [2,3]. Comparable to MERS-CoV and SARS-CoV-1 outbreaks, the current SARSCoV- 2 outbreak has characteristic features of rapid transmission among human beings to be classified as the largest global public health menacing to humanity in this century. The World Health Organization (WHO) had declared on March 2020 the COVID-19 outbreak a pandemic owing to the wide number of infectious cases with an accelerated mortality rate. High SARS-CoV-2 mutations and the appearance of several variants in different countries readily result in several challenges for the existing vaccines. The unprecedented swift outbreak of the COVID-19 pandemic urges the detection and development of innovative vaccines, antiviral agents with a high degree of safety and efficiency.
Overview on COVID-19 Therapeutic Options
However, plenty of therapeutic agents have been estimated for COVID-19 treatment, no antiviral drug has yet been demonstrated to be effective [4]. Recently, the Food and Drug Administration (FDA) has approved only one drug, remdesivir, to treat COVID-19 [5]. Remdesivir is a broad-spectrum antiviral of RNA-dependent RNA polymerase with in vitro suppressive activity against MERSCoV and SARS-CoV-1 [6-9]. Recent studies have been reported the potency of remdesivir to inhibit SARS-CoV-2 [10,11]. Furthermore, monoclonal antibodies such as casirivimab, imdevimab, and bamlanivimab have been authorized for emergency use by the FDA, assisting the immune system in SARS-CoV-2 recognition and attachment to respond more effectively to the virus [12,13]. Since publishing a detailed SARS-CoV-2 genome, several studies shed new light on innovative targets to support researchers and pharmaceutical industries in developing and producing potent SARS-CoV-2 therapies [14,15].
Current Approaches for covid-19 Therapy
There are two fundamental strategies for COVID-19 therapy relying on handling a single therapeutic agent for only one target or indicating conventional drug combinations for two or more targets of SARS-CoV-2. The first one is no longer propitious particularly in the case of SARS-CoV-2 infection because of massive variations and mutations in the viral genome as well as the entire evolutionary virulent factors of SARS-CoV-2 are still unknown along with the overwhelming number of patients and deaths. The second, exhibiting numerous serious complications and toxicities due to non-selectivity. Therefore, both of them are yet to be satisfactory for pandemic control.
The evolution of antiviral structural design based on RdRp SARS-CoV-2 and SARS-CoV-2 Mpro selective targeting
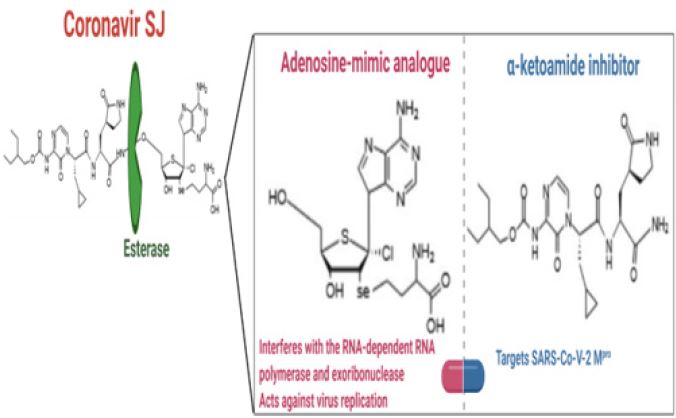
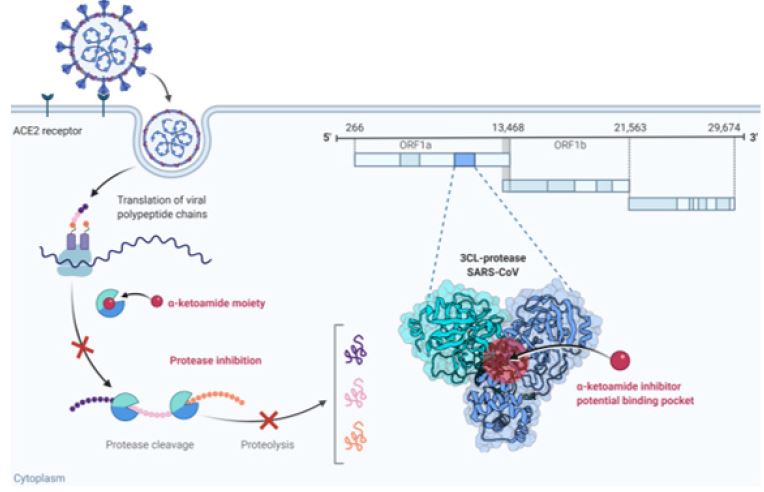
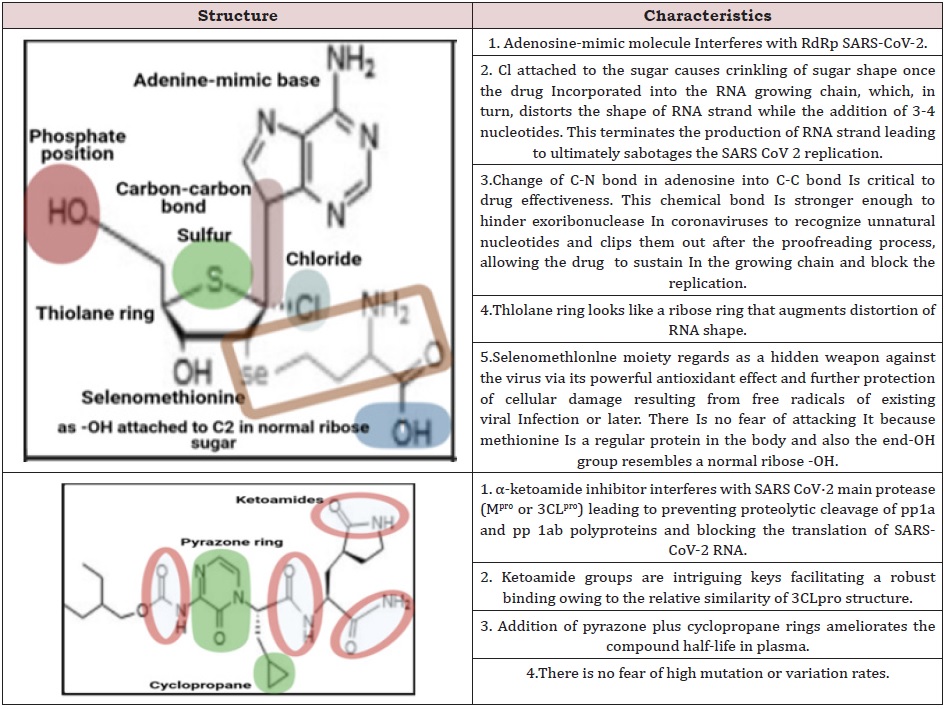
We discuss designing a prospective antiviral structure (Figure 1,2) that blocks the main biological processes of SARS-CoV-2, encounters viral resistance mechanisms, and ensures the safety of patients. Coronavir SJ could cleave once passed into the blood by esterase enzyme into adenosine-mimic analog and α-ketoamide inhibitor exerting robust SARS-CoV-2 inhibitory effects (Table 1); (Figure 3).
The formula of adenosine-mimic analog is C16H23N5O4SSeCl. Mechanistically, the main adenosine-mimic analog activity is relied on compromising the viral RNA-dependent RNA polymerase in infected cells, whereas it interferes with the proofreading function of exoribonuclease of newly synthesized RNA to excise nucleotide-based therapeutics that should not be integrated into the RNA leading to a reduction in SARS-CoV-2 RNA replication and production [16]. This compound is similar to adenosine nucleotide which is bioactivated within cells via kinases by the triphosphate’s addition for compromising RNA synthesis (not DNA synthesis), thus it is active against SARS-CoV-2 RNA. The most significant activity of this active metabolite triphosphate is being the interference with RNA-dependent RNA polymerase (RdRp) and exoribonuclease enzymes. This compound suppresses active sites in both SARSCoV- 2 enzymes (RdRp and exonuclease). This leads to a rising integration; retarded chain ends, and reduced excision (Figure 4).
On the other hand, an α-ketoamide inhibitor with the formula C24H35N6O6 overlaps with SARS-CoV-2 Mpro (also called 3CL), is the substantial protease characterizing with cleavage specificity that totally different to all human proteases, responsible for processing polyproteins pp1a and pp1ab that are translated from the viral RNA into different putative non-structural proteins (NSPS). Zhang and co-authors reported a recent study in science indicating the crystal structure of SARS-CoV-2 Mpro prompting researchers for creating and synthesizing innovative inhibitors for SARS-CoV-2 Mpro targeting [17]. Downregulation this main target would prohibit viral RNA replication and block the formation of structural proteins (Spike protein, Nucleocapsid protein, Membrane protein, and Envelope protein). This leads to the viral assembly via polyproteins cleavage preventing COVID-19 progression (Figure 4).
A two-sided therapeutic platform has been planned to reveal a promising therapeutic candidate with a state-of-the-art manner exerting the probability of potent and efficient inhibition of SARS-CoV-2. Coronavir SJ could provide a selectively favorable therapeutic inhibitor for SARS-CoV-2. This hypothesis displays new analogs of essential molecules targeting SARS-CoV-2 that could be incorporated into one chemical structure to provide us tremendous advantages for SARS-CoV-2 therapy (Figure 5). These scientific and technical merits encompass that Coronavir SJ is a prodrug thus it must be modified once administered in the body before it becomes an active drug. Prodrugs are regularly utilized for many reasons, including protecting a drug till reaching its location of action. This prodrug would provide two active drugs targeting two different sites of action with versatile functions. It is administered directly into the blood, accelerating its action and preserving it from major absorption and metabolic barriers. In addition, there is no fear of rapid mutation of SARS-COV-2 because this drug targets the backbone of the virus with a minor possibility of variation comparable with targets that regulate spike proteins production. Eventually, the existence of selenomethionine could represent a powerful antioxidant as a hidden weapon for extra protection from cellular damage resulting from the release of endogenous free radicals once viral infection incidence.
CONCLUSION
On the basis of SARS-Co-V2 pathogenesis and virulence, we hypothesise that two-sided targeting of both RdRp SARS-CoV-2 and SARS-CoV-2 Mpro harnessing new antiviral structural design to be a potent and selective inhibitor for SARS-CoV-2 without interference with animal or human enzymes or DNA, hence it is unlikely to be pharmacologically toxic. Although this design might be evidenced insufficient, we discuss that this therapeutic insight boosts several questions for upcoming research. Future scientific research is urgently needed to evaluate the potential therapeutic activity of Coronavir SJ on SARS-CoV-2.
Animal experiments and clinical trials should be performed to examine the in vivo effects of both adenosine-mimic analog and α-ketoamide inhibitor on SARS-CoV-2. Such beneficial frameworks could be exploited for further therapeutic studies and research purposes in the future. We believe that dense research efforts from scientists and researchers should be recommended to guarantee COVID-19 pandemic control and termination.
ACKNOWLEDGEMENTS
We acknowledge Bio Render for design support for the figures.
AUTHORS CONTRIBUTION
SAM and EIAS -conceived and designed the study, were responsible for the data acquisition, analysis, and interpretation. EIAS -responsible for the manuscript drafting. SAM – responsible for critical revision for important intellectual content. All the authors approved the manuscript final version submitted and all of the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17, 181-192.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) A novel coronavirus from patients with pneumonia in China. N Engl J Med 382: 727-733.
- Chen J, Qi T, Liu L, Ling Y, Qian Z, et al. (2020) Clinical progression of patients with COVID-19 in Shanghai. China J Infect 80(5): e1-e6.
- Madsen LW, Beigel JH, Tomashek KM, Dodd LE, Mehta AK, et al. (2020) Remdesivir for the treatment of Covid-19-final report. N Engl J Med 383: 1813-1826.
- FDA approves first treatment for COVID-19.
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, et al. (2020) Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11: 222.
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9(2): 00221-002218.
- Brown AJ, Cihlář T, Baric RS, Sheahan TP, Denison MR, et al. (2019) Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic delta coronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 169: 104541.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, et al. (2017) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9(396): eaal3653.
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30: 269-271.
- Wang Y, Zhou F, Zhang D, Zhao J, Du R, et al (2020) Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, doubleblind, placebo-controlled, multicentre trial. Trials 21: 422.
- Regeneron Pharmaceuticals, Inc. US Food and Drug Administration.
- Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab.
- Wang H, Li X, Li T, Zhang S, Wang L, et al. (2020) The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis 39(9): 1629-1635.
- WHO (2021) Genomic sequencing of SARS-CoV-2.
- Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello, et al. (2020) Current pharmacological treatments for COVID‐19: what’s next? Br J Pharmacol 177: 4813-4824.
- Zhang L, Lin D, Sun X, Drosten C, Sauerhering L, et al. (2020) Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368(6489): 409-412.
Article Type
Perspective
Publication history
Received Date: July 07, 2022
Published: July 20, 2022
Address for correspondence
Sameh Abdelmoneem Mohammed Ali, Faculty of Pharmacy, Department of Pharmacology and Toxicology, Beni-Suef University, Egypt
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Sameh AM, Esraa IAS. Two-Sided RdRp SARS-CoV-2 and SARS-CoV-2 Mpro Selective Targeting Approach for COVID-19 Pandemic Therapy. 2022- 4(4) OAJBS.ID.000471.
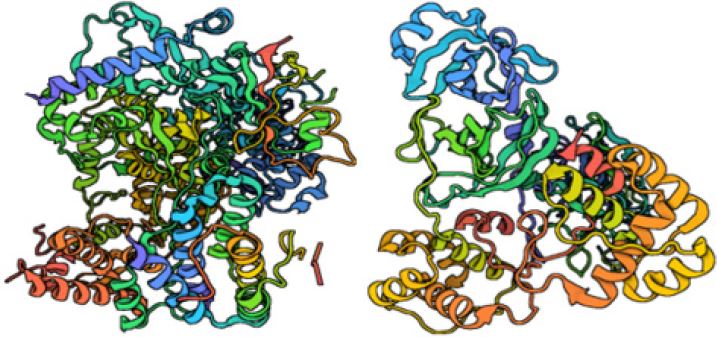
Figure 1: Three-dimensional structures of RdRp SARS-CoV-2 and SARS-CoV-2 Mpro (left to right).
Figure 2: Proposed Coronavirus SJ structural design as a potential antiviral for SARS-Co-V-2. Coronavirus SJ prodrug converts into its active metabolites (adenosine-mimic analog and α-ketoamide inhibitor) in the blood for molecular two-sided targeting of RdRp SARS-CoV-2 and SARS-CoV-2 Mpro in SARS-CoV-2 RNA genome.
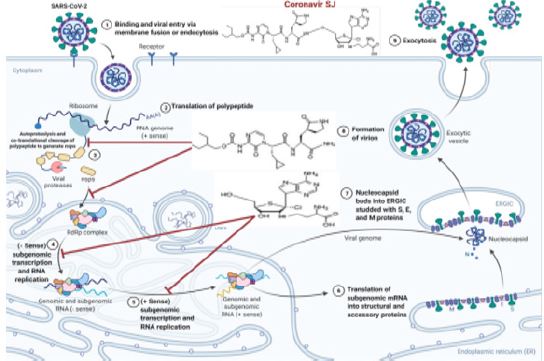
Figure 3: Proposed Coronavir SJ mechanisms of action across the infected cell as a potential antiviral for SARSCo- V-2. Coronavir SJ prodrug converts into its active metabolites (adenosine-mimic analog and α-ketoamide inhibitor) in the blood for molecular two-sided targeting of RdRp SARS-CoV-2 and SARS-CoV-2 Mpro in SARS-CoV-2 RNA genome. Adenosine-mimic analog blocks genomic replication and sub genomic transcription of main structural proteins of SARS-CoV-2. α-ketoamide inhibitor inhibits autoproteolysis process blocking polypeptide cleavage to produce different non-structural proteins (NSPs). Both thus could hinder SARS-CoV-2 to invade excessive human cells leading to curb SARS-CoV-2 progression.
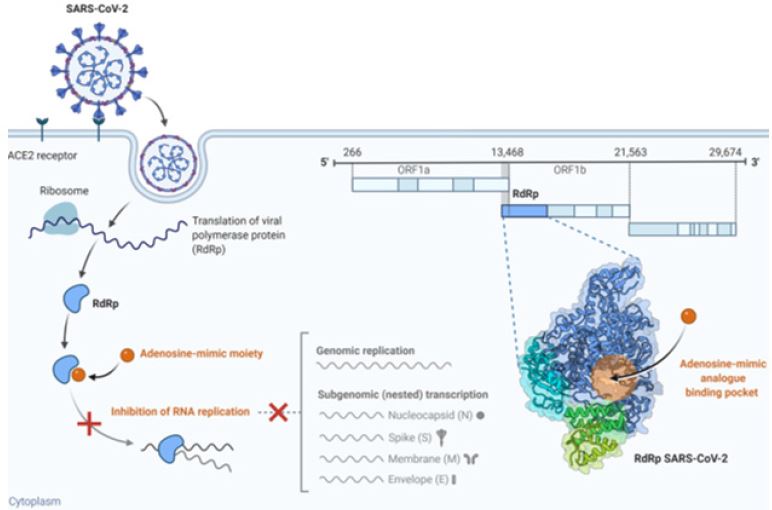
Figure 4: Proposed mechanism of action of adenosine-mimic analog against SARS-CoV-2 progression. adenosinemimic analog attaches to its binding pocket of RdRp-SARS-CoV-2, it initially inhibit RNA replication blocking genome replication and subgenomic transcription of nucleocaspid, spike, membrane, and envelope proteins, thereby control SARS-CoV-2 cellular invasion and progression.
Figure 5: Proposed mechanism of action of α-ketoamide inhibitor against SARS-CoV-2 progression. α-ketoamide inhibitor attaches to its binding pocket of SARS-CoV-2 Mpro, it could cause protease inhibition blocking polyproteins pp1a and pp1ab cleavage that are translated from the viral RNA into different putative non-structural proteins (nsps), thus preventing moving to genomic replication and subgenomic transcription of nucleocaspid, spike, membrane, and envelope proteins for control SARS-CoV-2 cellular invasion and progression.
Table 1: Key features of designed adenosine-mimic analog and α-ketoamide inhibitor against SARS-CoV-2.