Thrombotic Thrombocytopenic Purpura Secondary to a Covid-19 Vaccination: A Real Thread or Common Side Effect
ABSTRACT
Vaccination against COVID-19 has undoubtedly reduced the severity of the disease with a decrease in hospitalizations and health costs. However, since their introduction, many adverse events have been reported, the severity of which varies greatly. we will report the case of a thrombotic thrombocytopenic purpura acquired in a patient free of any pathology in the aftermath of COVID-19 vaccination.
KEYWORDS
COVID-19; Vaccination; TTP
INTRODUCTION
The COVID-19 pandemic has seen the development of different types of vaccines with different mechanisms of action, all of which aim to directly protect vaccinated individuals from severe disease progression and thus reduce ICU admissions and deaths. However, as with any pharmaceutical product, vaccines designated against the SARS-CoV-2 virus may cause adverse events in some individuals, including idiopathic acquired thrombotic thrombocytopenic purpura. Thrombotic thrombocytopenic purpura (TTP) is considered to be a rare multisystem disorder that was first described by Moschcowitz [1]. We present here the case of a 40 years old patient that was diagnosed with TPP days after receiving the COVID-19 vaccine.
CASE PRESENTATION
A 40-year-old patient with no medical or surgical history other than chronic smoking, was presented to the emergency department with many ecchymosis and fever.
The medical examination showed a conscious man with normal blood pressure 130/60 mmHg, elevated heart rate at 120 bpm, SpO2 at 98% at ambient air and fever: 39C°, with multiples ecchymosis on the torso, back and the upper and lower limbs.
The patient reported that the history of the disease date back to 3 weeks with progressive onset of headaches, arthralgias, myalgias, fever and generalized asthenia, the patient consulted for these symptoms and was put under vitamins and paracetamol but no improvement was observed. However, the patient mentioned that he received the second shot of the COVID-19 vaccine days before the appearance of the symptoms. On the light of these findings, the patient was transferred to the internal medicine unit for further examination. The blood test showed, thrombocytopenia at 11,000elmts/mm³ with regenerative normochromic anemia hemoglobin level at 5.2g/dl and reticulocyte level at 300,000elmts/mm³ and the presence of the schistocyte in the blood smear. The blood work-up showed direct bilirubin 12mg/l, LDH>1995 U/l, haptoglobin <0.08g/l with negative Coombs test. An ADAMTS 13 assay was performed and came back under <10%.
The hemostasis work-up showed a PT and ACT at the normal range, fibrinogen at 2.37g/l and the D-dimer levels were high >10000ug/l, the rest of was normal with no renal, hepatic disturbance. An infectious screening was carried out consisting of blood cultures, Sputum cytobacteriological exam, urine cytobacteriological exam, which came back negative. HIV, HBV and HCV serology were negative. And in order to look for an undiscovered neoplasia a CT-PET scan was performed and came back normal.
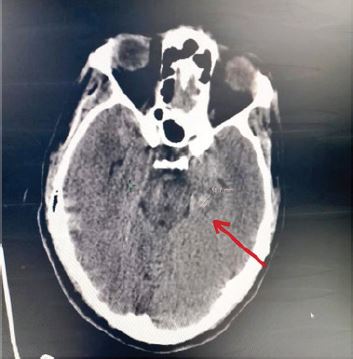
During his hospitalization, the patient got agitated, and presented a generalized seizure, a cerebral CT scan was carried out, which did reveal a small hemorrhagic lesion in the left hemisphere [Figure 1]. All clinical and biological arguments were in favor of vaccination-induced thrombotic thrombocytopenic purpura and the patient was transferred to the ICU unit for further management. During his time in the ICU, the management of his condition consisted on:
a) methylprednisolone 2mg /kg/day
b) transfusion of 4 RGCs and 4 FFPs
c) plasma exchange
d) rituximab 700mg/Day x 4 doses (D0, D8, D15, D21)
e) Vincristine: 2mg one dose
f) sodium valproate 500mg: 1cp/8h
g) phenobarbital 200mg: 1cp in the evening
DISCUSSION
Thrombotic thrombocytopenic purpura is commonly known as an autoimmune disease in which the immune system produces antibodies responsible for the destruction of ADAMTS13. The deficiency of this enzyme causes platelets to clot inappropriately in in the blood vessels, resulting in thrombocytopenia sets in with multiple organ failure [2].
TTP is most often acquired and more rarely inherited. The inherited form is the result of a mutation in the gene that codes for ADAMTS13 [3]. However, the acquired form can be divided into either the primary form, which has no obvious cause, or the secondary form, which is most often secondary to autoimmune disease, pregnancy, organ transplants, drugs, infections, tumors and other conditions [4]. The typical form of TTP is idiopathic acquired TTP with a sudden onset of asthenia, arthralgia and myalgia, as well as low back pain and abdominal pain, which can be mistaken for an infectious process. The five cardinal signs characterizing the clinical picture are: fever, neurological disorders, renal failure, mechanical hemolytic anemia and peripheral thrombocytopenia [5]. The diagnosis is based on a number of factors including thrombocytopenia, mechanical hemolytic anemia, the presence of schistocytes on peripheral blood smear with elevated LDH and low haptoglobin, but the specific diagnosis is based on the determination of the activity of ADAMTS 13 protein, which is very low [6]. Recently in the literature, many health professionals have reported cases of post-vaccination TTP secondary to vaccination against COVID-19 regardless of the type of vaccine used. The clinical presentation is similar to our patient. As far as we know, ten patients with TTP induced by the COVID-19 vaccine have been reported in the literature. Most of these situations occurred after the second dose of COVID-19 vaccine, and 7 patients were noted to have received the BNT162b2 vaccine. Caplacizumab was used in 6 patients, and complete remission was achieved in 8 patients [7].
CONCLUSION
The recent incidents described in the literature regarding COVID-19 vaccination have caused much debate regarding their use, however, no one can deny their effectiveness in reducing morbidity and mortality. TTP is a serious and sometimes fatal complication, but judging by the benefit/risk ratio, there are no arguments for suspending their use at the moment.
ACKNOWLEDGEMENT
AB, AZ, and CC examined the patient and drafted the manuscript. FS and ND evaluated the findings and gave important clinical opinions. BH and AB participated in the design of the case report and helped to draft the manuscript. All authors read and approved the final manuscript.
REFERENCES
- Moschcowitz E (1952) An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries; an undescribed disease. Am J Med 13(5): 567-569.
- Dorooshi G, Lalehzar SS, Nasri M, Meamar R (2020) Thrombotic thrombocytopenic purpura with conjunctivitis in a patient with coronavirus disease 2019 infection. Adv Biomed Res 9: 71.
- Kremer Hovinga JA, Lämmle B (2012) Role of ADAMTS13 in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2012: 610-616.
- Saha M, McDaniel JK, Zheng XL (2017) Thrombotic thrombocytopenic purpura: pathogenesis, diagnosis and potential novel therapeutics. J Thromb Haemost 15(10): 1889-1900.
- Iqbal S, Zaidi SZA, Motabi IH, Alshehry NF, AlGhamdi MS, et al. (2016) Thrombotic thrombocytopenic purpura - analysis of clinical features, laboratory characteristics and therapeutic outcome of 24 patients treated at a tertiary care center in Saudi Arabia. Pak J Med Sci 32(6): 1494- 1499.
- Tsai HM, Lian EC (1998) Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 339(22): 1585-1594.
- Hammami E, Lamarque M, Aujoulat O, Debliquis A, Drénou B, et al. (2022) Acquired thrombotic thrombocytopenic purpura after BNT162sb2 covid-19 vaccine: case report and literature review. Lab Med.
Article Type
Case Report
Publication history
Received Date: May 13, 2022
Published: May 26, 2022
Address for correspondence
Bouaiyda Ayoub, Department of Anesthesiology and Intensive Care, Mohammed V University, Morocco
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Chouikh C, Bouaiyda A, Azzouzi A, Mounir Kh, Fejjouji S, Doughmi N, Balkhi H, Baite A. Thrombotic Thrombocytopenic Purpura Secondary to a Covid-19 Vaccination: A Real Thread or Common Side Effect. 2022- 4(3) OAJBS.ID.000453.