Studying of Anthocyanins of Cerasus Vulgaris Mill. Species Spreading in the Territory of Nakhchivan Autonomous Republic and its Application as pH Indicator
ABSTRACT
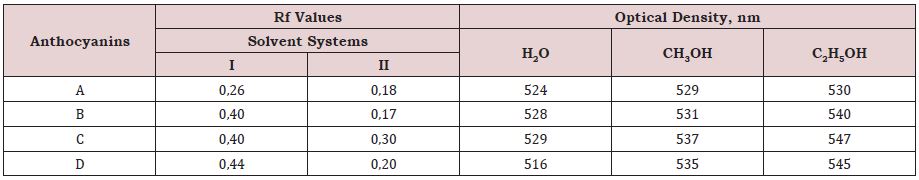
The color of anthocyanins changes not only according to their chemical structure, but also according to the pH and concentration of the medium. The most influential are their pH values for color and consistency. Extraction and purification of anthocyanin pigments from Cerasus vulgaris Mill. species was successfully carried out. According to the obtained Rf values and UV wavelengths, it was determined that component A was cyanidin 3-sophoroside, component B was cyanidin 3-glucosylrutinoside, component C was cyanidin 3-glucoside, and component D was cyanidin 3-rutinoside. Also UV spectrum of components were measured. The Rf value in the solvent system of component A was 0,26 and 0,18 in the II, and the UV wavelength was 524, 529, 530 nm, respectively, according to the relevant solvents. In the solvent system of component B, the Rf value was 0,40, in the II 0,17, and the UV wavelength was 528, 531, 540 nm, respectively, according to the relevant solvents. In the solvent system of component C, the Rf value was 0,40, in the second 0,30, and the UV wavelength was 529, 537, 547 nm, respectively, according to the relevant solvents. In the solvent system of component D, the Rf value was 0,44, in II - 0,20, and the UV wavelength was 516, 535, 545 nm, respectively, according to the relevant solvents.
KEYWORDS
P.cerasus; Phenolics; Phtochemical; Spectrophotometer; Anthocyanin; Pigment; UV wavelengths
INTRODUCTION
Plants are the most important source of compounds that are very useful for the human body, including natural antioxidants. Analysis of the chemical composition of plants by modern and more sensitive methods allows the identification and specification of new substances in them. Plants distributed in the flora of the Nakhchivan Autonomous Republic have a rich phytochemical composition, and therefore the study of the phytochemical composition of these plants is one of the important issues [1,2].
Anthocyanins are derived from the Greek words for flower (anthos) and blue (kianos) and are a group of water-soluble natural pigments for pomegranates, and radishes, their colors ranging from pink to purple. The literature shows that there are about 500 anthocyanins. This is why many fruits, vegetables and flowers can come in many different colours. The structure of the flavonoids that make up the anthocyanin class is determined by the presence of H, OH and OCH3 groups in rings A and B. Anthocyanins combine with one or more sugar molecules to form anthocyanins under different hydroxylated conditions. They are glycosides of anthocyanins. If these compounds are hydrolyzed and the glycosidic bond is released, the residue will be anthocyanin. This part is also called “aglycon” [3].
In the structure of phenolic compounds that form the aglycone part of anthocyanins, the blue color increases as the number of -OH groups increases, and the red color increases as the number of OCH3 groups increases. About 20 anthocyanins are known. 6 of them are widespread in fruits and vegetables. These compounds are pelargonidin, cyanidine, peonidine, dolphinidine, petunidine and malvidin [4]. The color of anthocyanins changes not only according to their chemical structure, but also according to the pH and concentration of the medium. The most influential are their pH values for color and consistency. They form purple-red at low pH and blue-green at high ph. Anthocyanins are light red in an acidic environment, purple in a neutral, blue-green in an alkaline, and blue in a highly alkaline condition. Anthocyanins are compounds with high colouring ability and therefore can be widely used as a natural colouring agent in the colouring of many foods. These compounds include alcoholic and non-alcoholic beverages, canned fruits, sweets, dry foods, dairy and flour products, etc. are used for food colouring [5,6]. It is widely accepted that fruits and vegetables have many healthful properties. Citrus fruits, including oranges, lemons, limes and grapefruits, are a principal source of such important nutrients. They contain vitamin C, dietary fibre, and other bioactive components such as carotenoids and flavonoids, which are suggested to be responsible for the prevention of degenerative disease [7].
Cerasus vulgaris species of Rosaceae family is a medicinal plant. This plant especially the fruits are used in urinary system to cure number of diseases such as urinary tract infection, nephrolithiasis, cystolithiasis, and dysuria in the USM. Since this was an important medicinal plant since long, and therefore, scientists were also curious to prove the pharmacological actions. Leaves are reddish, more in number and similar to yellow potato leaves. Flowers are white. Fruits are small, rounded and similar to grape which is attached to the smaller branch by thin and fine wood in the pair of bunch. There were more than fifty cultivars of sour cherry in England before the Second World War. Sour cherries require rich, well drained, moist soil for cultivation, although they demand more nitrogen, and water than sweet cherries. During spring, flowers should be protected, and trees weeded, mulched and sprayed with natural seaweed solution. This is also the time when any required pruning should be carried out. The tree of sour cherry is smaller than the sweet cherry. Its height is of 4-10m. Leaves are ovate, hard, stiff and rather abruptly pointed, minutely toothed; flowers white, in cluster of 2-5 on slender pedicles, 2-4cm long appearing with the leaves; fruits globose, 0,6-1,25cm in diameter, light red to nearly black, acid or sweet. Bark is bitter, astringe. Fruit is sour and sweetish [8].
MATERIAL AND METHODS
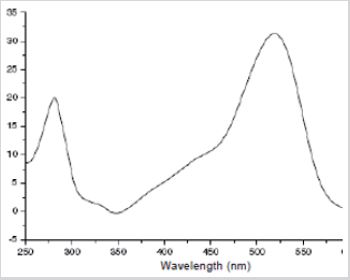
Cerasus vulgaris were crushed, and air-dry raw materials were extracted with hydrochloric acid solution of ethyl alcohol, with constant stirring. The raw material was extracted several times. The combined extracts were filtered through filter paper and concentrated on a vacuum rotary evaporator. The thickened extract was used for further research. The qualitative anthocyanin composition of the extract was determined by partition chromatography on paper. Fruit extracts were obtained using various solvents. Water, methanol and ethanol were taken as solvents. After the extracts were concentrated, column and thin layer chromatography were performed. Extracts were measured at between 200-700 nm by Hitachi U-2900 UV-VIS. Labconco Clear Drying Chamber with Valves 7443500. Thin layer chromatographic analysis has been carried with DC-fertigfolien ALUGRAM SİL G/ UV 254. Using paper chromatography in various solvent systems, it was determined that the anthocyanin complex from species consists of four components (A,B,C,D). Using preparative paper chromatography, all four components were isolated individually, their Rf was determined in various solvent systems and UV spectra were recorded. HAW, HCI-acetic acid-water (3:30:10); BAW, buthanol- acetic acid-water (4:1:5). The results of the studies are presented in table. The chromatogram was monitored at 518nm [5,6].
DISCUSSION OF THE RESULTS
Fruit extracts of the species were obtained and TLC were carried out. As a result of chromatography, 4 components were purified and separated. The systems used for thin-layer chromatography, the obtained Rf values and the spectral results are shown in Table 1. The table shows the Rf values of the species extract obtained in the two systems used in TLC chromatography and the wavelength values obtained by UV spectrophotometry.
RESULTS
Anthocyanins represent one of the most widely distributed classes of flavonoids in plants. The difference in chemical structure that occurs in response to changes in pH is the reason that anthocyanins are often used as pH indicator, as they change from red in acids to blue in bases. This unique property of these pigments has been exploited as an application of anthocyanins during the project work. According to the obtained Rf values and UV wavelengths, it was determined that component A was cyanidin 3-sophoroside, component B was cyanidin 3-glucosylrutinoside, component C was cyanidin 3-glucoside, and component D was cyanidin 3-rutinoside. As can be seen from the table, the Rf value of component A in the I solvent system was 0,26 and 0,18 in the II, the UV wavelength was 524, 529, 530 nm, respectively, according to the relevant solvents. In the solvent system of component B, the Rf value was 0,40, in the II 0,17, and the UV wavelength was 528, 531, 540 nm, respectively, according to the relevant solvents. In the solvent system of component C, the Rf value was 0,40, in the second 0,30, and the UV wavelength was 529,537,547nm, respectively, according to the relevant solvents. In the solvent system of component D, the Rf value was 0,44, in II-0,20, and the UV wavelength was 516, 535, 545 nm, respectively, according to the relevant solvents (Figure 1).
The application of purified anthocyanins were studied as natural pH indicator. The colour of Anthocyanin pigments changes according to the pH of the medium. The color of anthocyanins depends on the acidity of the medium. Juice of cherry was studied to change color in different environments. In HCl medium pH-1, in NaOH solution pH-8, in CH3COOH pH-1, in Na2CO3 solution pH-13 values were obtained and the results are shown in the Figure 2.
REFERENCES
- Rahimova SA (2016) Chlorophyll and carotenoids of fruits and leaves of Capparis herbacea L. (wild celery) International cooperation in the development of agrarian science, food security and environmental protection, 8th international scientific-practical conference. Azerbaijan State Agrarian University, Ganja, pp.141-145.
- Rahimova SA (2016) Coloring properties of metal complexes of some plant pigments in leaf and fruit extracts of Capparis herbacea L. (wild carob). Public Association for Support of Youth Scientific Research International Journal of Young Researchers, Baku pp. 238-242.
- Mzia D, Maia V, Indira D (2013) Phenol Compounds of Blackberry (Rubus caucasicus Focke and Rubus anatolicus L.). Fruit and Leaf 7: 539-546.
- Guliyev V B, Mansur H (1999) Flavonoids. Cagaloglu, Istanbul p. 370.
- Brouillard R, Cheminat A (1988) Flavonoids and plant color. Prog Clin Biol Res. 280: 93-106.
- Naczk M, Shahidi F (2004) Extraction and analysis of phenolics in food. Journal of Chromatography A 1054: 95-111.
- Ozgen M, Serce S, Kaya C (2009) Phytochemical and antioxidant properties of anthocyanin Morus nigra and Morus rubra Fruits. Scientia Horticulture 275-279.
- Kirakosyana A, Seymoura EM, Noon KR, Llanes DE, Kaufman PB, et al. (2010) Interactions of antioxidants isolated from tart cherry (Cerasus vulgaris) fruits. Food Chem 122: 78-83.
Article Type
Mini-Review
Publication history
Received date: April 21, 2021
Published date: June 02, 2021
Address for correspondence
Rahimova Sura Ali, Doctor of Philosophy in Biology, Institute of Bioresources of Nakhchivan Branch of NAS, Azerbaijan
Copyright
©2021 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Rahimova SA. Studying of Anthocyanins of Cerasus Vulgaris Mill. Species Spreading in the Territory of Nakhchivan Autonomous Republic and its Application as pH Indicator. 2021- 3(3) OAJBS.ID.000288.