SGLT-2 Inhibitors: A Useful Addition for Treatment of Heart Failure with Mildly Preserved and Preserved Ejection Fraction
ABSTRACT
Background: The use of sodium-glucose cotransporters type 2 (SGLT2) inhibitors is associated with reduction in cardiorenal
outcomes in patients with heart failure and reduced left ventricular ejection fraction (HFrEF).
Objective: To clarify the therapeutic role of SGLT2 inhibitors in patients with heart failure and mildly preserved ejection fraction
(HFmpEF) and heart failure with preserved ejection fraction (HFpEF).
Methods: Pubmed search until October 26, 2022. Search terms included: heart failure, SGLT2 inhibitors, hospitalization,
mortality, safety. Randomized clinical trials and guidelines of major societies were reviewed.
Results: 2 well-designed trials, the EMPEROR-Preserved and DELIVER trials, have shown that use of SGLT2 inhibitors was
associated with decrease cardiac events in patients with HFmpEF and HFpEF. In the EMPEROR-Preserved, empagliflozin 10 mg/d
decreased a composite primary outcome of cardiovascular (CV) death or hospitalization for heart failure (HHF) compared with
placebo, hazard ratio (HR) 0.79 (95% CI, 0.69-0.90, P<0.001). In the Deliver trial, dapagliflozin 10 mg/d decreased the primary
outcome of CV death or worsening HF, HR 0.82 (95% CI 0.73-0.92, P<0.001). The effects of empagliflozin and dapagliflozin on the
primary outcome were evident and statistically significant versus placebo after 13-18 days post randomization. In both Emperorpreserved
and Deliver trials, no significant effects on CV death were demonstrated. By pooling data from the 2 trials, the effects
of empagliflozin and dapagliflozin on CV death was close but did not reach statistical significance, HR 0.88 (95% CI 0.77-1.00,
P=0.052). Meanwhile, after pooling 2 dapagliflozin trials to include patients with HFrEF (DAPA-HF trial) and HFmpEF + HFpEF
(DELIVER trial), dapagliflozin significantly decreased CV death, HR 0.86 (95% CI, 0.75-0.98, P=0.02) and all-cause mortality, HR 0.90
(95% CI, 0.82-0.99, P=0.03). The CV effects of empagliflozin and dapagliflozin were consistent regardless of age, gender, presence or
absence of diabetes or atrial fibrillation. Yet, the effect of empagliflozin on decreasing HHF was attenuated in patients with baseline
left ventricular ejection fraction (LVEF) of ≥60% and disappeared at LVEF ≥65%. On the other hand, dapagliflozin effects on cardiac
outcomes remained consistent regardless of baseline LVEF. Both empagliflozin and dapagliflozin were generally well tolerated, with
rates of drug discontinuation due to adverse effects similar to those with placebo.
Conclusion: SGLT2 inhibitors should be the standard of care in patients with HFmpEF and HF pEF similar to their established
indication in patients with HFrEF. Until direct comparison between empagliflozin and dapagliflozin becomes available, dapagliflozin
should be the SGLT2 inhibitor of choice, particularly in patients with HFmpEF and HFpEF.
KEYWORDS
SGLT2 inhibitors; Heart Failure; Mortality; Ejection Fraction; Safety
INTRODUCTION
Accumulating evidence have shown that use of SGLT-2 inhibitors was associated with decrease in HHF and CV death in patients with HFrEF (defined as LVEF ≤40%) with and without diabetes [1,2]. Accordingly, current guidelines recommend SGLT2 inhibitors in patients with HFrEF to reduce HHF and CV mortality irrespective of presence of type 2 diabetes (class IA recommendation, i.e., strong recommendation, high-quality evidence). In patients with HFmpEF (LVEF 41-49%) and HFpEF (LVEF ≥50%), treatment options are limited [3]. The first study that suggested a role of SGLT2 inhibitors in treatment of HFpEF was the SOLOIST-WHF trial that evaluated sotagliflozin versus placebo in patients with diabetes and recent HF (n=1,222). In the latter study, sotagliflozin decreased HHF, urgent visits for HF, or CV death by an impressive 52% (HR 0.48, 95% 0.27- 0.86) in the subgroup of patients with LVEF ≥50%. This subgroup constituted 21% of the study population. Unfortunately, the SOLISTWHF was terminated prematurely after a median follow-up of 9 months due loss of funding [4].
More recently, 2 landmark trials, the EMPEROR-Preserved and DELIVER, were published. Both trials were specifically designed to examine the effects of empagliflozin and dapagliflozin, respectively on CV clinical outcomes in patients with HFmpEF and HFpEF [5,6]. The main purpose of this review is to provide a critical appraisal on the therapeutic role of the 2 SGLT2 inhibitors empagliflozin and dapagliflozin in patients with HFmpEF and HFpEF based on the findings of the EMPEROR-Preserved and DELIVER trials.
Overview Of the Emperor-Preserved and The Deliver Trials
The EMPEROR-Preserved and the DELIVER trials are 2 large multinational randomized trials that examined the effects of empagliflozin and dapagliflozin, respectively on CV events in patients with symptomatic HFpEF [5,6]. Overview and main results of the 2 studies are summarized in Table 1. Participants had New York Heart Association (NYHA) class II-IV and an LVEF of >40%, with evidence of structural heart disease (left ventricular hypertrophy or left atrial enlargement) associated with N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels of > 300 pg/ml and > 900 pg/ml in patients with atrial fibrillation. In general, inclusion and exclusion criteria of the 2 trials are similar with 2 differences. First, the DELIVER included patients with improved LVEF, i.e., those who had had a previous LVEF of < 40% but improved to > 40% at the time of enrollment [6]. Second, the cutoff of estimated glomerular filtration rate (eGFR) required for enrollment was different. Thus, patients were excluded from EMPEROR-preserved and DELIVER trials if their eGFR was < 20 ml/min/1.73 m2 and < 25 ml/ min/1.73 m2, respectively [5,6]. Patients were randomized into 2 equal groups to receive empagliflozin 10 mg/d and dapagliflozin 10 mg/d versus placebo in addition to standard care of HF [5,6]. The primary outcome in the EMPEROR-Preserved trial was a composite of CV death or HHF. In the DELIVER trial, the primary outcome was a composite of CV death or worsening heart failure. The latter included HHF in addition to urgent visit for heart failure (i.e., a non-scheduled visit to an office or emergency department for a main diagnosis of HF requiring intravenous diuretic therapy); [6]. Thus, the DELIVER trial added urgent visits for HF to the composite primary outcome. Secondary outcomes differ between the 2 trials and are outlined in Table 1.
Main Results of Emperor-Preserved and The Deliver Trials
In the EMPEROR-Preserved trial, over a median of 26.2 months, a primary outcome event occurred in 13.8% and 17.1% of patients randomized to empagliflozin and placebo, respectively; HR 0.79 (95% 0.69-0.90; P< 0.001) [5]. In the DELIVER trial, over a median of 2.3 years, the primary outcome occurred in 16.4% and 19.5% of patients randomized to dapagliflozin and placebo respectively, HR 0.82 (95% CI, 0.73-0.92; P< 0.001) [6]. Interestingly, in both trials, the decrease in the primary outcome events was mainly driven by the significant reduction in incidence of HHF. Thus, HR for the decrease in HHF were 0.73 (95% CI 0.61-0.88) and 0.77 (0.67- 0.89), with empagliflozin and dapagliflozin, respectively [5,6]. The difference in occurrence of CV death, HHF, or an urgent visit for heart failure between empagliflozin and placebo was recorded early becoming statistically significant at 18 days after randomization [7]. In the case of dapagliflozin, this difference occurred at 13 days post-randomization and was sustained at the end of follow-up [8].
Effect On Empagliflozin and Dapagliflozin on Cardiovascular and All-Cause Mortality
In EMPEROR-Preserved and DELIVER trials, the decreased risk of the second component of primary outcome, CV death, did not reach statistical significance (Table 1). Similarly, neither empagliflozin nor dapagliflozin significantly decreased all-cause mortality (Table 1); [5,6]. After pooling the results of both trials (n=12,251), the effect of empagliflozin and dapagliflozin on all-cause mortality remained non-significant, HR 0.97 (95% CI, 0.88-1.06), and reduction in CV death was close to statistically significant, HR 0.88 (95% 0.77-1.00; P =0.052) [9]. Meanwhile, in a meta-analysis of the 2 trials of dapagliflozin including patients (n=11,007) with HFrEF (DAPA-HF trial) and HFmpEF + HFrEF (DELIVER trial), dapagliflozin significantly decreased CV mortality (HR 0.86, 95% CI 0.75-0.98, P=0.02) as well as all-cause mortality (HR 0.90, 95% CI 0.82-0.99), P=0.03) [10]. Taken together, the above observations suggest that dapagliflozin, but not empagliflozin, may decrease CV death and all-cause mortality in patients with HF across the whole spectrum of EF i.e HFrEF, HFmpEF and HFpEF.
Subgroup Analysis
The CV effects of empagliflozin and dapagliflozin on cardiac outcomes did not vary in subgroups classified by age, gender, body mass index, presence or absence of diabetes or atrial fibrillation, degree of frailty, or background use of CV medications [5,6,11- 16]. However, a remarkable difference between empagliflozin and dapagliflozin emerged in terms of baseline LVEF. Thus, in case of dapagliflozin, its effects on CV outcomes did not differ across different values of LVEF ranging from ≤30% up to ≥ 60% [17]. However, in case of empagliflozin, the risk reduction in CV death and HHF was attenuated with LVEF ≥60% and is totally lost with LVEF≥65% [5-18].
Patients With Improved LVEF
Improved LVEF refers to those patients with a previous LVEF < 40% that improved to > LVEF > 40% [3]. Patients with improved EF deserves particular attention due to the following causes. First, despite their growing prevalence, they are usually excluded from trials of HFpEF [19]. Second, even when LVEF return to normal range, these subjects may have worse clinical outcomes than patients with no history of HF [19]. Third, preliminary data suggest that withdrawal of pharmacological HF drugs was associated with relapse in 36% of patients within 6 months of withdrawal [20]. Accordingly, current guidelines recommend that patients with improved EF should continue HF treatment [3]. Patients with improved LVEF were excluded from the EMPEROR-Preserved trial but were allowed to participate in DELIVER trial forming 18.3% of the study population [6-21]. Importantly, results from the DELIVER trial showed that the CV benefit of dapagliflozin was consistent in the subgroup of patients with improved EF [6].
Effects On Symptoms of Heart Failure
Dapagliflozin and empagliflozin improved health-related quality of life symptoms of HF as evaluated by the Kansas City Cardiomyopathy Questionnaire (KCCQ) scored from 0 to 100, with higher scores indicating fewer symptoms and physical limitation. The amelioration in the KCCQ score was modest (mean improvement < 5 points) but statistically significant. Thus, mean placebo-corrected difference between baseline and month 8 in KCCQ total symptom score was in favor of dapagliflozin group, 2.4 points (95% CI, 1.5-3.4) [6]. With empagliflozin, corresponding difference was 1.53 (95% CI 0.85-2.40) at 32 weeks and 2.07 (95% 1.15-2.99) at 52 weeks [22].
Effects Of Empagliflozin on Renal Function
Effect of empagliflozin on renal function was among the prespecified secondary outcomes of the EMPEROR-Preserved trial but was not reported in the DELIVER trial [5,6]. The EMPERORpreserved showed that the rate of decline in the eGFR was slower in the empagliflozin group than in the placebo group (-1.25 versus -2.62 ml/min/1.73 m2 per year; P<0.001) [5]. In addition, Feirrera et al. [23] performed post-hoc analysis of pooled data from the EMPEROR-REDUCED that evaluated patients with HFrEF and the EMPEROR-Reduced trials (total patient number 9,673). They found that empagliflozin, as compared with placebo, reduced the incidence of macroalbumimuria, defined as spot urine albumin-tocreatinine ratio (UACR) >300 mg/g, by 19% (HR 0.81; 95% CI 0.70- 0.94; P=0.005) [23]. The latter effect was consistent in patients with baseline LVEF ≤40% and >40%, with eGFR ≤ 60 and > 60 ml/ min/1.72 m2, and those with and without diabetes [23].
Safety of Empagliflozin and Dapagliflozin
Both empagliflozin and dapagliflozin were well tolerated. Proportions of patients with serious adverse effects and those who discontinued treatment due to adverse effects were similar to those randomized to placebo (Table 1); [5,6]. In EMPEREORPreserved, some adverse effects were reported more commonly with empagliflozin compared with placebo including hypotension (10.4% vs 8.6%), urinary tract infections (UTI) (9.9% vs 8.1%), and genital infections (2.2% vs 0.7%); [5]. These adverse effects were not mentioned in DELIVER trial, except for “serious” UTI, which occurred equally in 1% of dapagliflozin and placebo groups [6]. It was reassuring that frequency of acute renal injury and hypoglycemia was not increased with empagliflozin or dapagliflozin [5,6].
Differences Between Empagliflozin and Dapagliflozin
to empagliflozin in 3 aspects. First, as mentioned above, when data from Deliver trial and DAPA-HF trial were pooled to encompass the whole range of LVEF, dapagliflozin was associated with significant 14% reduction in CV death and 10% in all-cause death [10]. On the contrary, no mortality benefit was shown with empagliflozin [5]. Second, the cardiac benefits of dapagliflozin extends to patients with LVEF >60%, whereas in case of empagliflozin, this benefit was attenuated with LVEF >60%, and is totally lost with LVEF > 65% [5-18]. Third, in terms of safety, hypotension, UTI, and genital infections were reported more frequently with empagliflozin compared with placebo, whereas these adverse effects were not mentioned with dapagliflozin. The reasons for this discrepancy between empagliflozin and dapagliflozin are unclear but might be related to differences in patients’ characteristics and presence of some dissimilarities in drug properties. Therefore, until head-tohead trials for direct comparison of empagliflozin and dapagliflozin become available, dapagliflozin should be the agent of choice in patients with HF in general, and those with HFpEF in particular.
LIMITATIONS
Although the EMPEROR-Preserved and DELIVER trials are welldesigned, they suffer from several limitations [5,6]. First, owing to the multiple exclusion criteria, patients are relatively healthier than patients in real-life. For instance, patients with eGFR <20 ml/ min/1.73 m2 have yet to be studied. Second, while Asians were fairly represented in the 2 trials (13-20%), other ethnic groups are underrepresented. In fact, less than 5% of subjects were Blacks, and proportions of Hispanics were not reported. Therefore, it is still unknown whether SGLT2 inhibitors have similar or different effects in various ethnic groups and minorities. Third, some types of cardiomyopathies were excluded such as infiltrative cardiomyopathy (e.g., amyloidosis, sarcoid), genetic or obstructive hypertrophic cardiomyopathy.
CONCLUSION AND CURRENT DIRECTIONS
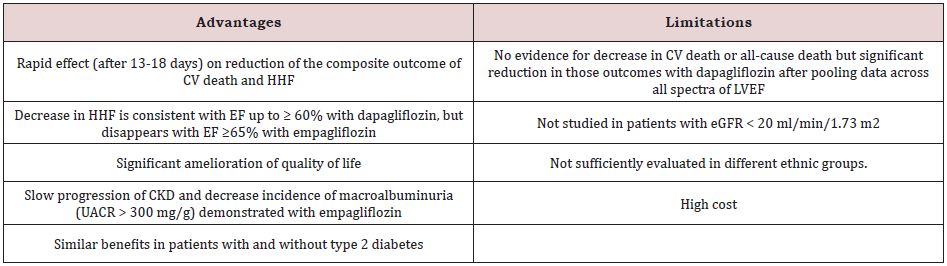
Empagliflozin-Preserved and DELIVER trials provide strong evidence that the use of SGLT2 inhibitors in patients with HFmpEF and HFpEF may be associated with significant reduction in HHF [5,6]. These 2 categories of HF are of utmost need for new medications that convincingly reduce CV morbidity and mortality. Most recent guidelines published before the release of DELIVER Trial recommend SGLT2 inhibitors as class 2aB recommendation, i.e moderate-strength recommendation, moderate quality evidence, in patients with HFmpEF and HFpEF [3]. After the release of results of the DELIVER Trial, it is likely that this recommendation will be upgraded to class 1A, like that in HFrEF. While neither empagliflozin nor dapagliflozin individually demonstrated decreased CV death and all-cause mortality in patients with HFmpEF and HFpEF over a median follow-up of up to 2.3 years, longer follow-up (e.g., 5 years) is needed to clarify the effects of SGLT2 inhibitors on these 2 outcomes. Nevertheless, pooling data of the 2 dapagliflozin trials, DAPA-HF and DELIVER, suggest that dapagliflozin may be associated with significant 14% reduction in CV death and 10% in all-cause death [10]. In addition, contrary to empagliflozin, dapagliflozin cardiac benefits persist at LVEF > 60%. Moreover, the safety profile might be more favorable with dapagliflozin (Table 1); [5,6]. Therefore, until head-to-head trials become available, dapagliflozin may be the SGLT2 inhibitor of choice for use in patients with HF. Advantages and limitations of SGLT2 inhibitors for treatment of HFmpEF and HFpEF are summarized in Table 2.
REFERENCES
- Murray JJVMC, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, et al. (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21): 1995-2008.
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, et al. (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383(15): 1413-1424.
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, et al. (2022) AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145(18): e876-e894.
- Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, et al. (2021) Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 384(2): 117-128.
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, et al. (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385(16): 1451-1461.
- Solomon SD, Murray JJV, Claggett B, Boer RA, David DM, et al. (2022) Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 387(12): 1089-1098.
- Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, et al. (2021) Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: emperor-Preserved Trial. Circulation 144(16): 1284-1294.
- Vaduganathan M, Claggett BL, Jhund P, Hernandez AF, Inzucchi SE, et al. (2022) Time to clinical benefit of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified secondary analysis of the deliver randomized clinical trial. JAMA Cardiol e223750.
- Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, et al. (2022) SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomized controlled trials. Lancet 400(10354): 757-767.
- Desai AS, Jhund PS, Claggett BL, Vaduganathan M, Miao ZM, et al. (2022) Effect of dapagliflozin on cause-specific mortality in patients with heart failure across the spectrum of ejection fraction: a participant-level pooled analysis of dapa-hf and deliver. JAMA Cardiol e223736.
- Böhm M, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. (2022) Empagliflozin improves outcomes in patients with heart failure and preserved ejection fraction irrespective of age. J Am Coll Cardiol 80(1):1- 18.
- Butler J, Filippatos G, Siddiqi TJ, Ferreira JP, Brueckmann M, et al. (2022) Effects of empagliflozin in women and men with heart failure and preserved ejection fraction. Circulation 146(14): 1046-1055.
- Adamson C, Kondo T, Jhund P, de Boer RA, Honorio JWC, et al. (2022) Dapagliflozin for heart failure according to body mass index: the deliver trial. Eur Heart J ehac 44(41): 4406-4417.
- Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, et al. (2022) Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation 146(9): 676-686.
- Butt JH, Jhund PS, Belohlávek J, Boer RA, Chiang CE, et al. (2022) Efficacy and safety of dapagliflozin according to frailty in patients with heart failure: a prespecified analysis of the deliver trial. Circulation 146(16): 1210-1224.
- Oyama K, Raz I, Cahn A, Goodrich EL, Bhatt DL, et al. (2022) Efficacy and safety of dapagliflozin according to background use of cardiovascular medications in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol 7(9): 914-923.
- Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, et al. (2022) Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med 28(9): 1956-1964.
- Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, et al. (2022) Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J 43(5): 416-426.
- Wilcox JE, Fang JC, Margulies KB, Mann DL (2020) Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. J Am Coll Cardiol 76(6): 719-734.
- Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, et al. (2019) Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomized trial. Lancet 393(10166): 61-73.
- Anker SD, Siddiqi TJ, Filippatos G, Zannad F, Ferreira JP, et al. (2022) Outcomes with empagliflozin in heart failure with preserved ejection fraction using deliver-like endpoint definitions. Eur J Heart Fail 24(8): 1400-1405.
- Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, et al. (2022) Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the emperor-preserved trial. Circulation 145(3): 184-193.
- Ferreira JP, Zannad F, Butler J, Filippatos G, Pocock SJ, et al. (2022) Association of empagliflozin treatment with albuminuria levels in patients with heart failure: a secondary analysis of emperor pooled. JAMA Cardiol e222924.
Article Type
Review Article
Publication history
Received Date: October 27, 2022
Published: December 02, 2022
Address for correspondence
Nasser Mikhail, Department of Medicine, OliveView- UCLA Medical Center, United States
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Nasser Mikhail. SGLT-2 Inhibitors: A Useful Addition for Treatment of Heart Failure with Mildly Preserved and Preserved Ejection Fraction. 2022- 4(6) OAJBS.ID.000524.
Table 1: Trials of empagliflozin and dapagliflozin on heart failure with preserved ejection fraction.
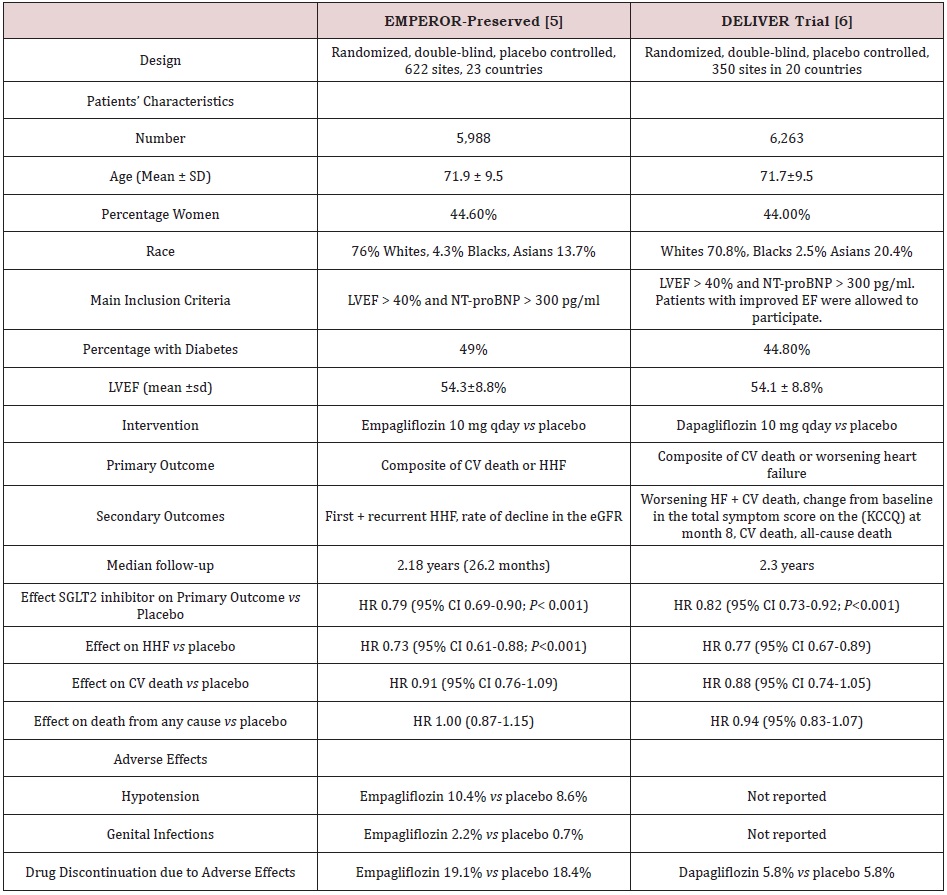
Abbreviations: NT-ProBNP: N-Terminal Pro-B Type Natriuretic Peptide; LVEF: Left Ventricular Ejection Fraction; CV: Cardiovascular; HHF: Hospitalization for Heart Failure; EGFR: Estimated Glomerular Filtration Rate; KCCQ: Kansas City Cardiomyopathy Questionnaire; SGLT2: Sodium-Glucose Cotransporter 2; HR: Hazard Ratio
Table 2: Trials of empagliflozin and dapagliflozin on heart failure with preserved ejection fraction.