Sanitation of Cages for Mice: Frequency, Type of Floor and Animal Stress
ABSTRACT
The issue of stressors such as the frequency of cleaning and the preferred type of flooring for mice in animal facilities is a major discussion in Laboratory Animal Science. Our objective was to carry out an experimental design, where through the growth of the Swiss Webster Outbred Stock (from 3 weeks of age) it was possible to evaluate the number of boxes exchange and the type of floor that would not be a stressor factor for the animals. Our results show that the repetition of changing boxes twice a week does not promote stress in the animals. Mice prefer wood shavings over pine flakes. Since the pine flakes promote in mice susceptible to stress the elevation of reactive oxygen species in the cerebral cortex. In addition, the pine flake floor has the ability to retain ammonia up to the 6th week of the animal’s life, when the system is used once a week. Thus, we suggest that the lineage, the number of animals per box and the maintenance of two changes per week be evaluated. However, we suggest using pine flakes only if mixed with wood shavings, regardless of the number of changes.
KEYWORDS
Laboratory animal; Welfare; Change box
INTRODUCTION
The mouse is one of the most used species for didactic and scientific purposes. The origin of the mouse occurred 14 million years ago by rodents that inhabited the region between India and Pakistan and generated the genus Mus, the subgenus Mus, the species Mus musculus and several subspecies. This speciation was likely related to their migration, colonization, and commensal relationship with humans. Thus, a feature that stands out in the mouse is its adaptive capacity and social flexibility, always seeking reproductive success through food provision, territorialism and the formation of small groups, usually polygamous. Thus, we can say that the mouse kept its wild behavior in its genetic characteristics, such as competition and interpersonal aggressiveness, as well as its close relationship with humans and its high adaptability to new environments, especially when related to the ease of food supply [1].
BEA’s commitment to mice kept in animal facilities is mainly linked to factors:
a. human, users of laboratory animals;
b. environmental, related to the housing and maintenance of
animals and
c. intrinsic to the animal, related to the behavior and biology of
each species [2,3].
The environment can compromise the BEA when it does not allow the animals, such as the mouse model, to express the natural behavior of their species. Therefore, it is necessary to emphasize that the animals are kept in a space restriction to minimize environmental variables that compromise the reproducibility of the test results [4,3]. Brazilian Guide to the Production, Maintenance or Use of Animals in Teaching or Scientific Research Activities. In item 2.2. Procedures for the area of production and maintenance of rodents and lagomorphs and its sub-item 2.2.4.
Determines that the activity of transferring animals from a cage where they were (dirty cage) to a new cage (clean cage) is called Box Exchange. This task should not be a mechanical act, but a moment for the application of the ethological management and observation of the animal, since it is in the exchange that changes in the animal’s health status are perceived. The frequency of exchange is a consequence of:
a) Physical structure of the facility where the animals are kept,
the material offered for bedding, number of animals in the cage and
physiological state of these animals;
b) In open cages with good air exchange (10 to 20 air changes
per hour), in polygamous mating systems, two changes per week
can be performed;
c) In microisolators, with animals in monogamous mating
and good bedding material, the frequency can reach 10 to 14 days
without changing;
So, our working hypothesis is to determine what would be the ideal number of box changes for each strain, sex and age of mice kept in ventilated racks in a vivarium, as well as the measurement of ammonia concentration in the environment, that is, considering cages open with 10 to 20 air changes per hour. These environmental conditions are representative in most Brazilian animal facilities [5- 9].
MATERIALS AND METHODS
Animals
Swiss Webster outbred stock, males from CEMIB/UNICAMP. They will be requested upon arrival at 3 weeks of age and kept in the LBC/LITEB – IOC vivarium for acclimatization. We will start all tests with infant animals (4th week of life - sdv) and with duration of each group with the same time, 30 days, that is, at the end we will be with adult animals, in the 8th week of life.
Non-invasive parameters: After the first week of adaptation, before the beginning of each trial (composed of 4 animals/cage) of each strain, the following parameters will be performed in the 4th sdv. All assays will be in duplicates.
Clinical assessment: Body, hair, skin, body posture, mouth and teeth and urogenital region assessment. Realization of body weight (grams); Food consumption (water and feed) consisting of the difference in volume and weight between what was offered and what was withdrawn for each group.
Tail Suspension Test
The TSC will be used to characterize the activity and reaction of the individual animal when subjected to a stressful situation, that is, the animals will be suspended by the final third of the tail in the upper part of the apparatus and their periods of agitation and immobility will be recorded for 5 minutes. Immobility will be characterized by the absence of twisting, rotating or attempts to lift the body.
Filming
All rehearsals will be filmed, evaluated and archived by an IP Robot Camera 3 Antennas, Wireless Wifi Wireless Hd with Night Vision (Infrared). Camera with motion alert system, infrared, where you can watch night images. WiFi connection, with total practicality and comfort, allowing you to monitor through the application on your cell phone, tablet, computer and notebook in high resolution.
Use of the “Trapeze”
The filming will always be carried out 48 hours after the microenvironment is changed. At that moment, whatever the time of microenvironment change, we will count the number and time for which the animals used the “Trapeze” available with Environmental Enrichment in the Cage.
Use of the “Trapeze”
Ammonia Measurement
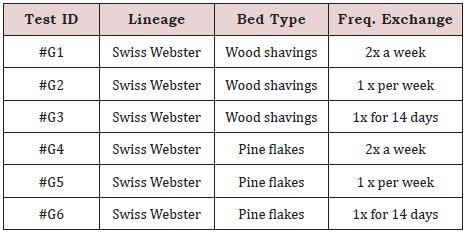
For the acquisition of data on ammonia concentration, temperature, humidity and luminosity, we will use the measurement by the Arduino Mega system daily (it shows all the values on its small LED screen). This device has a sensor that will be permanently inside the box and we will use 3 pieces of equipment, relating to a Set of different changes in the microenvironment. Then, this device will be placed next to the shelf in all the groups with their different exchange schemes and the value in parts per million (ppm) ≥ 25 ppm daily and the other environmental data will be measured (Table 1).
RESULTS
Our preliminary results demonstrate that mice prefer the flooring offered with wood chips (shavings) when compared to pine flakes. In addition, the offer of pine flakes causes hypoactivity in the animal during the Tail Suspension Test in relation to animals with a wood chip floor. In addition, they use the Trapeze less, object of environmental enrichment of the playful type. Changing boxes twice a week is viable up to the 8th week of life for the Swiss Webster, with a number of 4 in the cage. Regardless of whether the floor is shavings or pine flakes. The ammonia concentration at this time is ≥ 25 ppm.
These results are corroborated when there was only one exchange per week. Both for the use of wood shavings or pine flake as flooring, in the 6th week of life, in both types of flooring, the ammonia content in the cage was higher than 30 ppm. Another important factor is that changing the floors twice a week does not stress the animals. We evaluated the expression of reactive oxygen species in the cortex of the animals and observed that the ROS levels in the animals that remained on wood shavings were low. However, the animals that were allocated on the pine flake floor showed an elevation of 20 to 30% in ROS levels.
DISCUSSION AND CONCLUSION
This is a hotly debated topic in Laboratory Animal Science. Many believe that the lower the number of exchanges, the lower the stress on the animal. Thus, the most efficient pine flakes in absorbing ammonia would be the best type of flooring. However, we cannot forget that mice in laboratories are highly social. Even the relationship between the animal and the handler is a form of environmental enrichment for the animal, increasing its quality of life. Our preliminary results demonstrate that the number of boxes exchange (with adaptation) can be performed up to twice a week without being a stressor for the animal.
However, despite the efficiency of pine flakes in relation to ammonia concentration, we indicate that the ideal flooring for mice in the laboratory is performed through a mixture between pine flakes and wood shavings. In addition, the number of box exchanges will depend on the strain, sex, age and number of animals in the cage, but the exchange twice a week is not a stressor for mice in the house facilities.
REFERENCES
- Costa SMD, Rossi MID, Evagelista AA, Oliveira G (2019) Origin, phylogeny and natural behavior of mice: what is their influence on welfare during their maintenance in the house facilities? Am J Biomed Sci & Res 5(5).
- Appleby MC (2011) Animal Welfare, 2ª edição, Wallingford, England.
- Furtado AK, Oliveira GM, (2018) Biometric analysis related to the importance of the well-being of mice and the influence on the results of scientific trials. Resbcal São Paulo 6(2): 111-128.
- Spangenberg EM, Keeling LJ (2015) Assessing the welfare of laboratory mice in their home environment using animal-based measures - a benchmarking tool. Lab Anim 50(1): 30-38.
- Evangelista AA, Costa SM, Rossi MI, Oliveira GM (2019) Wild mouse & laboratory mouse historical aspects, genetic selection and welfare. R Soc bras Ci Anim Lab 7(2): 122-129.
- Kuzel, MA, Oliveira GM, Demarque KC, Rangel JA, Rodrigues FV, et al. (2013) Study of the hierarchy of swiss webster mice through the use of interconnected cage systems (SGI). Resbcal 2(1): 49-60.
- Gamble MR, Clough G (1976) Ammonia build-up in animal boxes and its effect on rat tracheal epithelium. Lab Ani 10(2): 93-104.
- Rolim Wesley JR (2020) Development of a monitoring system for two factors: temperature, humidity, ammonia and luminosity in the breeding room of the IPEN animal farm. Master’s Dissertation - graduate program in nuclear technology (applications). Institute of Nuclear Research, São Paulo, Brasil.
- (2016) Resolução Normativa Nº 33. Baixa o Capítulo “Procedimentos - Roedores e Lagomorfos mantidos em instalações de instituições de ensino ou pesquisa científica, Brasil.
Article Type
Case Report
Publication history
Received Date: September 02, 2022
Published: September 29, 2022
Address for correspondence
Oliveira GM, Setor de Ciência em Animais de Laboratório, Instituto Oswaldo Cruz, Brasil
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Brück MG, Machado RM, Fragoso VM, Pessanha SV, Oliveira GM. Sanitation of Cages for Mice: Frequency, Type of Floor and Animal Stress. 2022- 4(5) OAJBS.ID.000492.