Review of Vitamin A Structural Analogues and their Pharmacokinetic Parameters
ABSTRACT
Vitamin A and its structural analogues belong to the retinoid family of chemicals (usually known as vitamers of Vitamin A). The goal of the review was to assess the different generations of retinoids, their structural analogues, pharmacokinetic properties, and medicinal applications to pave way for future research. This research looked at online journals from Pubmed, Research Gate, Elsevier, Plos One, Scopus, Springer, Google Scholar, Hindawi, African Journals Online, Lancet, and others. The numerous classes of retinoids were revealed in the review. Retinoids are best known for their role in embryo development and vision, via rhodopsin production. The first generation of retinoids are particularly beneficial in the prevention of measles, blindness, gene therapy, growth and development, conception, management of skin, neck, and head tumors, and chronic acne, as well as chronic dermatitis. The second-generation retinoids are useful for various skin diseases, severe resistant psoriasis, photoaging, skin wrinkles, cutaneous T-cell lymphoma, plaque psoriasis, and acne, while the third-generation retinoids are applicable topically in minor acne, keratosis, and mycosis. Finally, fourth-generation retinoids are used topically in acne and congenital ichthyosis. They possess distinctive mechanism of action, different pharmacokinetic parameters, and adverse effects. Thus, can be employed as lead compounds in the development of medicinally important molecules.
KEYWORDS
Vitamins; Retinoids; Vitamin A; Absorption; Distribution; Metabolism; Elimination
INTRODUCTION
Vitamins are organic molecules that enable an organism’s metabolism to function properly and are essential micronutrients. Most organisms cannot synthesize these essential nutrients, thus acquired through food and dietary supplements. Vitamins tend to be complex molecules, but there are very few single molecules Maton et al. [1]. According to the World Health Organization (WHO), there are thirteen vitamins Harvard Health [2], including retinoids (vitamin A), thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folic acid (B9), cobalamins (B12), ascorbic acid (C), calciferol WHO [3]; Ogechukwu et al. [4].
Biochemically, vitamins carry out different functions. In the case of vitamin A, it acts to promote cell growth and differentiation. Vitamin D functions are similar to those of biological enzymes in regulating mineral metabolism. Vitamin B complexes also act as coenzymes and enzyme precursors. The antioxidant properties of vitamin E and C are well documented Bender [5]. Deficiency of these vitamins, as well as excessive intake, can cause mild to serious illnesses. Retinoids regulate a variety of biochemical functions and activities, including epithelial cell growth, bone and tissue differentiation, cell proliferation regulation, immune stimulation, and tumor suppressor activation Kiser et al. [6]. Four (4) generations of retinoids have been identified based on their discovery and approval dates Food and Drug Administration [7]. Thus, this review aimed to assess the various retinoids, their structural Analogues, pharmacokinetic parameters, and medicinal applications.
METHODS
Data were retrieved from online and offline platforms related to Vitamin A vitamers (generations of retinoids), their structural Analogues, possible synthetic pathways, food and animal sources, stability profiles, pharmacokinetic parameters, medicinal applications, and methods of the assay. Journals from Pubmed, Research gate, Elsevier, Plos One, Scopus, Springer, Google scholar articles, Hindawi, African Journals Online, and Lancet, among others, were used for the review. Also, textbooks, conference proceedings, etc., were used in the review.
RESULTS AND DISCUSSION
Human metabolic functions necessitate fat-soluble vitamin A and its structural analogues. Among these are retinol, retinaldehyde (retinal), retinoic acid, and several provitamins A carotenoids (β-carotene); Blaner [8]. According to some studies, vitamin A has a range of roles in the body, including embryonic development, immune response maintenance, and eyesight. It joins opsin to generate rhodopsin, a light-absorbing molecule required for low-light and color vision Wolf [9]. Retinol, either condensed as retinol or retinylester, and carotenoids such as α-carotene, β-carotene, γ-carotene, and β-cryptoxanthin, which operate as provitamin A in herbivores and omnivores with the enzymes for their biotransformation, are all food sources of vitamin A Wu et al. [10]. Data obtained from the literature search revealed about four (4) different generations of retinoids (Vitamin A vitamers or analogues); FDA [7]. Tretinoin (retinoic acid), isotretinoin, retinal, retinol, and alitretinoin are among the first-generation retinoids, which are classified as Vitamin A vitamers due to their ease of conversion from retinol to retinal. The 2nd to 4th-generation retinoids is difficult to convert to retinol, retinal. Etretinate and its metabolite, acitretin, are in the second generation; bexarotene, adapalene, and tazarotene are in the third generation; and trifarotene is in the fourth generation Chelstowska et al. [11]. The retinoids have a hydrophobic molecular structure with a polyene side chain, cyclic, and polar end-end groups. A conjugated system generated by alternating C=C in the polyene ring gives retinoids their color (yellow, orange, or red). As a result, retinoids can function as chromophores. The polyene chain and end groups are alternated to form numerous generations of retinoids FDA [9]. Inflammatory skin disorders, acne, and psoriasis are often treated with retinoids National Psoriasis Foundation [12]. Toxic effects such as skin lesions, alopecia, malaise, pseudotumor cerebri, hepatosplenomegaly, bleeding, and even mortality occur following elongated use of retinoids. Some antimalarial medicines, such as proguanil, have been shown to affect retinoids’ systemic homeostasis Haldar et al. [13]. Furthermore, etretinate and isotretinoin are not recommended for usage during pregnancy due to the risk of CNS, craniofacial, and cardiovascular teratogenic abnormalities Choi et al. [14].
The First-generation retinoids
These include retinoic acid (tretinoin), isotretinoin, alitretinoin retinol, and retinal. They bind to several retinoid receptors owing to the flexibility imparted by their broken carbon-carbon double and single bonds Zasada [15].
Retinol: Retinol or Vitamin A1 (Figure 1), is a fat-soluble vitamin, obtained from food (fish) and other supplements. It is known to prevent xerophthalmia, a deficiency associated with vitamin A (American Society of Health-System Pharmacists-ASHSP [16]. It is useful in extreme Vitamin A deficiency conditions, thus reducing complications related to measles ASHSP [16]. Retinol is well tolerated at a normal dose, but high doses could lead to hepatomegaly, and dry skin British national formulary [17]; ASHSP [16]. Various enzymes in the body convert retinol to retinal and then to retinoic acid, although it is not relatively safe during pregnancy due to a biochemical chain of events Squires [18].
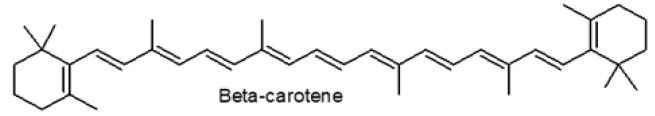
As a result of either a trans or cis configuration, retinoids have various geometric isomers. Retinol is created when β-carotene is broken down (Figure 2). The β-carotene molecule is cleaved at the central carbon-carbon double bond by the β-carotene-15,15’- monooxygenase enzyme, generating an epoxide that is further oxidized, forming two centrals OH groups which are successively condensed by NADH-dependent enzyme (retinol-dehydrogenase) producing retinal (an aldehyde), and further conversion to retinol Dewick [19].
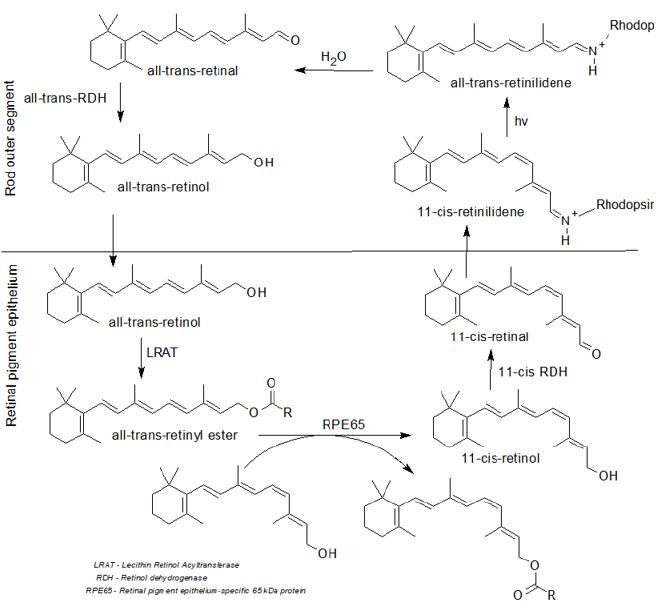
Protein-RPE65 converts retinol to 11-cis-retinal in the retinal epithelial pigment, which is subsequently delivered to photoreceptor cells in the retina via opsin proteins, which starts the visual cycle (Figure 3). A photon is translated to an electrical signal in the retina, which is where the visual cycle begins Leskov et al. [20]. Opsin, a G-protein coupled receptor protein that includes the chromophore 11-cis-retinal, aids this action. When the opsin receptor is covalently coupled to 11-cis-retinal via a Schiff base linkage, it forms retinylidene protein Ebrey [21]. 11-cis-retinal absorbs light and photoisomerizes to all-trans-retinal, changing the geometry of the opsin and triggering signal transduction cascades that lead to the closure of the cyclic GMP-gated cation channel and hyperpolarization of the photoreceptor cell in the early stages of the visual cycle Hsu et al. [22]. The shift in molecular conformation caused by light absorption alters the arrangement of rhodopsin, the low-light vision pigment. After photoisomerization and dissociation from the opsin protein, the all-trans-retinal is reduced to all-trans-retinol, which is subsequently returned to the retinal pigments epithelium to be recharged. Before RPE65 converts it to 11-cis-retinol, LRAT esterifies it.
Finally, it is oxidized to 11-cis-retinal before being attached to opsin in the rod outer segment and creating new functional rhodopsin (vision pigment) Arshavsky et al. [23]. This is why, although the effect on one’s vision is minor, taking meals or supplements enriched with vitamin A allows one to see even in the dark Leskov et al. [20]. Like other vitamin A analogues, retinol absorbs in the GIT completely. Preparations of retinol or its esters that are miscible with water are absorbed in GIT more rapidly than oily solutions. Following an oral dose of retinol in an oily solution, plasma steady-state takes 4-5 hours, while peak plasma concentrations take 3–4 hours for water-miscible formulations Buss et al. [24]. In smaller concentrations, it is stored as retinyl-palmitate in the liver, lungs, kidneys, retinas, adrenal glands, and intraperitoneal fat. Vitamin A is stored in the human body for several months to two years. It binds to retinol-binding protein (RBP), is not easily passed via the placenta, and is found in milk D’Ambrosio et al. [25]. Retinyl-ester-hydrolases break down retinol esters in the small intestine mucosa, producing free retinol. Retinol enters the intestinal absorptive cells via a passive diffusion route. It is reesterified to retinyl-palmitate by lecithin retinol-acyltransferase (retinyl palmitate), then incorporated into chylomicrons and secreted into the lymphatic system Institute of Medicine.
Retinol is conjugated with glucuronic acid; the β-glucuronide undergoes enterohepatic circulation and oxidized to retinal and subsequently retinoic acid. Retinoic acid undergoes decarboxylation and further conjugation with glucuronic acid. It is excreted in urine and feces via the biliary elimination pathway. It is highly stable in tight, light-resistant container ASHSP [26]. The Scavenger receptor class B type 1 (SRB1 or SCARB1), which is a membrane transporter protein, encoded by the SCARB1 gene in humans, aids the absorption of β-carotene in the enterocyte cells. These receptor levels increased during vitamin A deficiency. When vitamin A levels are optimal, SCARB1 and β-carotene-15,15’- monooxygenase (encoded by the BCMO1 gene), are responsible for the radial cleavage of β-carotene to retinal Wu et al. [27]; Blaner [8]. Absorbed carotene is either directly absorbed into chylomicrons or transformed to retinal and subsequently retinol before being linked to RBP2. After a meal, the liver absorbs around two-thirds of the chylomicrons, while the rest is transported to peripheral tissues. Tissues in the periphery can also convert chylomicron-carotene to retinol Green [28]; Blaner [8].
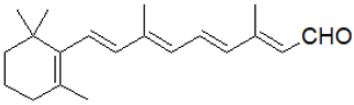
Retinal: The retinal dehydrogenase enzyme converts alltrans retinoic acid to retinal, which is a polyene chromophore. The chemical root of visual phototransduction (light detection and vision) is retinal (Figure 4), which is coupled to opsin proteins (National Center for Biotechnology Information - NCBI, 2022). The irreversible oxidation breakdown of carotenoids produces retinal (RAL); (Von et al. [29]; Woggon [30]. The retina possesses unique physiological and anatomical barriers Barar et al. [31]. Retinal is absorbed through the corneal Gaudana et al. [32]. The blood-ocular barrier (BOB) maintains the fluid composition, and aqueous humor and also controls the flow of aqueous humor, thus maintaining optimum ocular pressure. Physiological barriers such as turnover of tear, drainage in the nasolacrimal region, and blinking, while anatomical barriers like a blood-aqueous barrier; BAB, corneal stroma, epithelium, lymph flow, conjunctival blood, tear drainage, etc., affect retinal pharmacokinetics Agrahari et al. [33].
Retinoic acid (tretinoin): Retinoic acid directly regulates over 500 genes via RARs after successive diffusion into the cell nucleus Blaner [8]. It is used in acute promyelocytic leukemia Fenaux et al. [34]. Retinoic acid enhances vitamin A1 actions, which are necessary for cellular development and growth Duester [35]. The retinoid-X-receptor (RXR) is a DNA heterodimer that harbors the retinoic-acid-receptors (RARs) in the retinoic acid response elements (RAREs) locus. The all-trans-retinoic acid ligand interacts with the RAR, causing structural changes that stimulate or inhibit the transcription of nearby genes. Moutier et al. [36]. RARs regulate the differentiation of cells by mediating the transcription of a variety of genes Venkatesh et al. [37]. One of the target genes in animals is the RAR gene, which increases the retinoic acid response Wingender [38]. Moutier and colleagues discovered a shift in how retinoic acid receptors - retinoid X receptors (RARRXR) identify the genome from primarily 2 and 5 nucleotides (DR2 and DR5) to a more complicated form with a different halfsite spacing of the DNA binding motif in a comprehensive report. Moutier et al. [36]. The body produces all-trans-retinoic acid in two steps: retinol dehydrogenase transforms retinol to retinaldehyde, and retinaldehyde dehydrogenase converts retinaldehyde to all-trans-retinoic acid Duester [35]. To prevent toxicity, alcohol dehydrogenase and CYP2B6 enzymes metabolize excess all-transretinol Molotkov et al. [39].
Male rats administered all-trans-retinoic acid but without vitamin A1, suffered hypogonadism and infertility due to retinoic acid production deficits in the testes, according to a previous study. A comparable treatment resulted in infertility in female rats due to fetal resorption caused by a lack of embryonic retinoic acid synthesis Moore [40]; Van et al. [41]. Because aldehyde dehydrogenase (ALDH1A2, or RALDH2) catalyzes retinoic acid synthesis in the testes, inhibiting this enzyme could be a method of producing male contraceptives, as retinoic acid is required for spermatogenesis Kean [42]. In another study, when rats were supplemented with just all-trans-retinoic acid but no all-trans-retinol or retinal, no growth-stunting or epithelial-damaging effects were recorded (US National Research Council [43]; Chelstowska et al. [11]; Teresa et al. [44]. Retinoic acid or All-trans-retinoic acid (ATRA) is essential throughout life, but most critical during gestation Cunningham [45].
Isotretinoin: Isotretinoin is an isomer of tretinoin (retinoic acid). It was originally designed as a chemotherapy agent for leukemia. Isotretinoin is used in acne and skin neck, and head cancers. It has been reported to increase aminotransferase concentration in the blood Layton [46]; LiverTox [47]. A hyphenated technique, High-Performance-Liquid Chromatography- Electrospray Ionization mass spectrometry (HPLC-ESI-MS) has been used in the determination of isotretinoin in plasma using acitretin as an internal standard (IS), Wu et al. [27]. It is a known teratogen Choi et al. [14]. The correlations between isotretinoin and erectile dysfunction as well as reduced libido are well documented Alliance Pharmaceuticals [48]. Various spermatogenesis abnormalities, such as oligospermia, have also been reported European Medicines Agency [49]; (Figure 5 & 6).
Isotretinoin is a non-psychiatric medicine that has been linked to depression and is also among the top ten drugs used to attempt suicide Wysowski et al. [50]; Ludot et al. [51]. Dry eyes are prevalent during treatment, because of isotretinoin’s apoptotic influence on the meibomian glands; known to cause ocular impairment. As a result of this, some people have acquired contact lens sensitivity Brelsford [52].
Isotretinoin induces apoptosis in different cells, initiated from the meibomian glands, hypothalamic, and hippocampus cells Lambert [53]; Kremer et al. [54]; Sakai et al. [55]; Griffin et al. [56]; Nelson et al. [57]. It has a low affinity for RAR and RXR, but it can be transformed into metabolites that serve as RAR and RXRnuclear receptor agonists intracellularly Layton [46]. A previous study suggested that isotretinoin in the skin may amplify lipocalin production, which reduces sebum production via the induction of apoptosis in sebaceous glands while exhibiting an antimicrobial effect on Cutibacterium acnes Nelson et al. [58]; Wachter [59]. By inhibiting the enzyme, telomerase-reverse transcriptase (TERT) and telomerase, isotretinoin has been proven to prevent cellular immortalization and cancer Pendino et al. [60].
A possible biochemical basis for depression associated with isotretinoin use involves reduced cellular metabolism of the frontal lobe orbitofrontal cortex (OFC), which has been linked with headaches Bremner et al. [61]. Headache is often reported alongside neuropsychiatric symptoms, especially depression Wysowski [62]. Other psychological side effects, such as depression, may occur in people who are susceptible to isotretinoin’s CNS effects Bremner et al. [61]. In the central nervous system, isotretinoin binds to dopaminergic receptors (DRs); Kontaxakis et al. [63]. This affects dopaminergic neurotransmission by altering the geometry of DRs and reducing dopaminergic activity, which has been connected to psychiatric diseases such as depression. It is also known to enhance 5-HT1A receptor expression, thereby blocking serotonin release, and hence disrupting the serotonergic system. It also increases reuptake and decreases serotonin synaptic availability by upturning the conversion of the serotonin-transporter-protein (SERT-P); Borovaya et al. [64].
When taken with a high-fat meal, isotretinoin is better absorbed FDA [65]. Isotretinoin binds to plasma albumin proteins 99.9% of the time. Isotretinoin metabolites 4-oxo-isotretinoin, retinoid acid, and 4-oxo-retinoic acid have all been measured in plasma Brazzell [66]. Isotretinoin levels in the blood decrease after oral treatment, with a half-life of 90 hours. The metabolites, as well as their conjugates, are then excreted in the urine and feces FDA [67]. Alitretinoin or 9-cis-retinoic acid is an isomer of tretinoin. It is used as an antineoplastic agent, indicated for the management of skin lesions in AIDS-related Kaposi’s sarcoma and chronic hand eczema Ruzicka et al. [68].
Alitretinoin: Alitretinoin is believed to be the endogenous binding site for RXR, but it also activates the RAR Dawson [69]. In vitro, CYP3A4 and other cytochrome P450 enzymes oxidize it and convert it to 4-hydroxy-9-cis-retinoic acid and 4-oxo-9-cis-retinoic acid, however, the predominant metabolite discovered in vivo was 4-oxo-9-cis-retinoic acid, which is also isomerized to tretinoin Nelson et al. [70]. This enzyme’s plasma levels can be affected by any inhibitor or inducer. Following topical administration, it is not appreciably absorbed systemically; the elimination halflife is 2-10 hours, and it is eliminated in urine (64 %) and feces (30 %), Bidstrup et al. [71]. The common side includes headache, hypertriglyceridemia, decreased high-density lipoprotein, hypercholesterolemia, rash, pain, itchiness, anaphylactic reactions, hypersensitivity, depression, mood changes, suicidal thoughts, decreased night vision, etc., Panretin [72]; (Figure 7).
Second Generation Retinoids
Similar to the first-generation retinoids, the second-generation retinoids are also able to bind to several retinoid receptors due to their structural flexibility Khalil et al. [73]. Etretinate is a synthetic derivative of vitamin A, widely used in the management of skin diseases, affecting its keratinization Kaplan et al. [74]. Because the molecule is extremely lipophilic and is retained and released from adipose tissue, its effects can reappear after a lengthy period. It can be detectable in plasma for up to three years after treatment and is protein-bound to >99 percent Vahlquist et al. [75]. It has a low therapeutic index and a long elimination half-life of 120 days, making dosing difficult Mutschler [76]. Etretinate has been replaced with acitretin, a free acid (deprived of the ethyl ester). Despite its lower lipophilicity and a half-life of 50 hours, acitretin is largely metabolized in the body to etretinate Mutschler [76].
Etretinate: Etretinate is a teratogen, which means it can cause birth problems even after you’ve stopped using it Happle et al. [77]. It may also obstruct children’s bone growth Halkier-Sørensen et al. [78]. Individuals who received etretinate therapy are not fit to donate blood for the following 2-3 years Becker et al. [79]. It may induce bone or joint pain, stiffness, generalized idiopathic skeletal hyperostosis, muscular or abdominal cramps, dry, burning, itchy eyelids, atypical bruises, and other side effects after injection Kaplan [74]. Etretinate was approved to treat chronic psoriasis but was withdrawn in most countries due to the high teratogenic effects Qureshi et al. [80]; (Figure 8).
Geiger et al. [81] examined expectant mothers that were placed on etretinate therapy. In the animal model, neither a safe nor a hazardous dose was determined. Women who were exposed to retinoids before or during pregnancy had their data evaluated. When the medicine is given during pregnancy, there is a chance of spontaneous abortion or congenital deformity, according to the statistics (first trimester). After therapy, the risk was minimal because the number of anomalies did not appear to exceed that seen in the general population Geiger et al. [81].
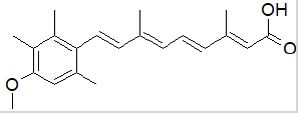
Acitretin: Acitretin is used in severe relapsing psoriasis Zito [82]. It is only used in the most severe situations because of its severe negative effects. It attaches to nuclear receptors, which control gene expression. They decrease cell reproduction by inducing keratinocyte differentiation and reducing epidermal hyperplasia Rendon [83]. Following oral dosing, acitretin is readily absorbed and broadly disseminated, with therapeutic effects visible within 2 to 4 weeks Pilkington [97]. Teratogenicity, headache, itching, flaky skin, reddish eyes, dry and swollen lips, thirst, cystic acne, or alopecia are some of the side effects Webmd [85]. Acitretin’s half-life is about 48 hrs and can be reversely metabolized to etretinate (with a long half-life), thus not safe for women planning a pregnancy in the following three years after use Almond-Roesler [86]; (Figure 9).
Third Generation Retinoids
They are less flexible than first and second-generation retinoids, and as a result, interact with fewer retinoid receptors (RR).
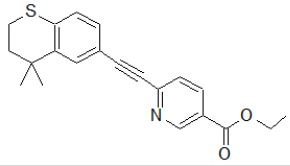
Tazarotene: Tazarotene is an acetylenic retinoid and a Vitamin A analog NCBI that is used to treat acne (usually with dapsone or clindamycin), Kolli et al. [87], plaque psoriasis, and photoaged or photodamaged skin ASHP [88]. It is contraindicated in pregnancy because it is a known teratogen. Some common adverse effects include skin redness, itchiness, and burning Mukherjee et al. [89]. It is selective for RAR- and RAR-like retinoic acid receptors and affects the differentiation and proliferation of epidermis keratinocytes by upregulating filaggrin expression and downregulating keratinocyte transglutaminase, ornithine decarboxylase enzymes, epidermal growth factor receptor, etc., Duvic et al. [90]; Heath [91]. About 99% of the active metabolite (tazarotenic acid) in the blood binds to plasma proteins (albumin), Tang-Liu [92]. The volume of distribution of tazarotene is about 26.1 L/kg, while that of tazarotenic acid is 1.97 L/kg (Tang-Liu, 1999). With a half-life of 16 to 19 hours, tazarotene is removed from the body in equal amounts through feces and urine Foster et al. [93]; Marks [94]; Menter [95]. The synthesis of tazarotene is via the Friedel-Crafts alkylation reaction route. Dimethylallyl bromide is alkylated with thiophenol, producing thioether. The thiopyran is obtained through cyclization of the alkene with corresponding polyphosphoric acid (PPA). In an aluminum chloride medium, the methyl ketone derivative is created via acylation with acetyl chloride. Enol-phosphate (intermediate) is produced when the enolate interacts with diethyl-chlorophosphate. The removal of diethyl-phosphite in the excess base gives rise to equivalent acetylene. Tazarotene is formed when the anion attaches to ethyl-6-chloronicotinate, and the chlorine atom is expelled (Figure 10).
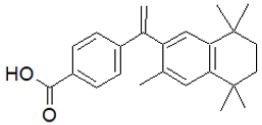
Bexarotene: Bexarotene, a third-generation retinoid, is useful in the management of mycosis, psoriasis, photoaging, and skin wrinkles Stefanaki [96]; Mukherjee et al. [89]; Kafi et al. [97]; Mayo Clinic [98]. It is an antineoplastic agent approved for cutaneous T-cell lymphoma treatment Gniadecki et al. [99]; Yuan et al. [100]. It has also been tried clinically and used for lung and breast cancer Dragnev et al. [101]; Esteva et al. [102]. The most common side effects include skin reactions, thyroid anomalies, leucopenia, headache, weakness (that is suspected to be a result of RXR-mediated downregulation of thyroid-stimulating hormone), hypercholesterolemia, hyperlipidemia, and hypothyroidism Brunton et al. [103]; Shahid et al. [104]. It is > 99 percent proteinbound, metabolized by the hepatic enzyme (CYP3A4) has an elimination half-life of around 7 hours, and is largely removed through the hepatobiliary system. Only 1% of the total is unaltered in the urine Bibi [105]. Concomitant use with ketoconazole, which is a CYP34A inhibitor, may raise plasma levels. It may also induce CYP3A4, lowering cyclophosphamide levels in the blood Borse et al. [106]. Bexarotene selectively activates the RXRs, leading to cell differentiation and apoptosis Rowe [107]; Dawson [69]. It also has anti-angiogenic effects and inhibits cancer metastasis Qu [92]. RARs control cell differentiation and proliferation, while RXRs control cell death Brunton et al. [103]; (Figure 11).
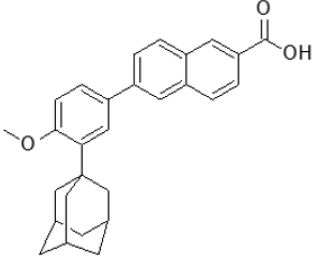
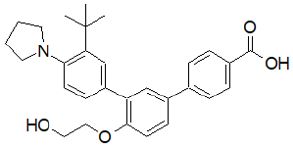
Adapalene: Adapalene is used primarily in mild to moderate acne and is off-label in the treatment of keratosis pilaris Rolewski [108]. Adapalene has been demonstrated in studies to be equally effective as other retinoids while causing less discomfort Tolaymat et al. [109]. Because adapalene is more stable than photodegradable tretinoin and tazarotene, it can be used in conjunction with benzoyl peroxide Tolaymat et al. [110]. It’s frequently used as a first-line treatment for comedonal acne Asai et al. [111]. Despite long-term use, topical adapalene has been shown to have poor systemic absorption and low blood levels (0.025 mcg/L), implying that there is a low risk of injury to a breastfeeding newborn but not to apply to a breastfeeding mother’s nipple Drugs and Lactation Database [112]; (Figure 12).
It improves the efficacy of topical clindamycin while also raising the risk of side effects Wolf et al. [113]; Jain [114]. Adapalene has a limited absorption rate through the skin. In a study of six acne patients who were given 2 g of adapalene once a day for five days as a cream applied to 1,000 cm2 of skin, no detectable 0.35 ng/mL of the medication was discovered in the plasma Piskin [115]. Adapalene is a highly lipophilic substance that easily permeates hair follicles and absorbs within 5 minutes of application Tolaymat et al. [116]. It binds to β and γ nuclear RARs Piskin [115]. These complexes then bind to the RXR, inducing gene transcription, thus controlling the downstream proliferation and differentiation of keratinocytes Piskin [115]; Tolaymat et al. [116]. Adapalene has been reported to suppress the inflammatory response and inhibits lipoxygenase and production of prostaglandins from arachidonic acid, hence it is considered an anti-inflammatory molecule. Retinization is a common temporary side effect when starting retinol therapy. It is best described by the redness of the skin, irritation, dryness, and possibly burning or itching during the first four weeks of treatment or retinol application; however, it usually goes away after four weeks Mukherjee et al. [89].
Fourth-Generation Retinoids
Trifarotene: Trifarotene (4th generation retinoid), approved in 2019; binds selectively to the RAR-y receptor. FDA [67]. Trifarotene is used in the topical management of acne vulgaris Cosio et al. [117]. It is a fourth-generation retinoid and a selective RAR-γ agonist Scott [118]. It was permitted for inborn ichthyosis management in FDA [7]. In the United States, trifarotene is designated for the topical management of acne vulgaris Brumfiel et al. [119]; Trifarotene [120] for acne. Trifarotene has been reported to be effective in skin infections, especially those relating to keratinocytes and it is easily metabolized by microsomes (hepatic cells), implying a higher level of safety. Trifarotene (0.01 %) has been proven to possess antiinflammatory, anti-pigmenting, and comedolytic properties when applied topically. Gene expression investigations revealed that stress response, epidermal transformation, proliferation, retinoic acid biotransformation, proteolysis, skin moisturizing, cellular transport, and adhesion are all potently stimulated by trifarotene Aubert et al. [121]; [122-131]; (Figure 13).
CONCLUSION
The hallmark medicinal uses of the retinoids are embryo development and vision, via the production of rhodopsin. The firstgeneration retinoids are vital in measles prevention, vision, gene replication, visual phototransduction, growth and development, severe acne and some forms of cancers, and chronic eczema. The second-generation retinoids are useful in various skin diseases, including severe resistant psoriasis, third-generation retinoids are applied topically in mild-moderate acne, keratosis, mycosis, psoriasis, photoaging, skin wrinkles, cutaneous T-cell lymphoma, plaque psoriasis, and acne, as well as photodamaged skin, while fourth-generation retinoids are used topically in ichthyosis of congenital origin, as well as acne vulgaris. The side effects and adverse reactions of some are of clinical concern, hence can be used as lead compounds in the development of medicinally important molecules.
REFERENCES
- Maton A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, et al. (1993) Human biology and health. Englewood Cliffs, New Jersey, USA.
- Harvard Health Publishing (2009) Listing of vitamins. Harvard Health.
- WHO (2004) Vitamin and mineral requirements in human nutrition (2nd edn). World health organization and food and agriculture organization of the United Nations. pp. 340-341.
- Ogechukwu LN, Samuel JB, Oyeintonbara M, Ezinne SI, Nnamdi MA, et al. (2022) The usefulness of herbal medicines in the prevention and management of coronavirus disease-2019 (COVID-19) and its symptoms: A review. Journal of Pharmacognosy and Phytochemistry 11(2): 68-78.
- Bender DA (2003). Nutritional biochemistry of the vitamins. Cambridge University Press, UK.
- Kiser, Philip D Golczak, Marcin Palczewski, Krzysztof (2013) Chemistry of the retinoid (Visual) cycle. Chemical Reviews 114(1).
- FDA (1999) Trifarotene Orphan Drug Designations and Approvals. U.S. Food and Drug Administration (FDA).
- Blaner WS (2020) Vitamin A. In Marriott BP, Birt DF, VA Stallings, AA Yates (Eds.), Present Knowledge in Nutrition, 11th edn, Academic Press, London, United Kingdom. P. 73-92.
- Wolf G (2001) The discovery of the visual function of vitamin A. The Journal of Nutrition. 131 (6): 1647-1650.
- Wu L, Guo X, Wang W, Medeiros DM, Clarke SL, et al. (2016) Molecular aspects of β, β-carotene-9’, 10’-oxygenase 2 in carotenoid metabolism and diseases. Exp Biol Med (Maywood). 241(17): 1879-1887.
- Chelstowska S, Widjaja Adhi M A, Silvaroli J A, Golczak M (2016) Molecular basis for vitamin a uptake and storage in vertebrates. Nutrients 8(11): 676.
- National Psoriasis Foundation (2010) Psoriasis.org.
- Haldar K, Bhattacharjee S, Safeukui I (2018) Drug resistance in Plasmodium. Nat Rev Microbiol 16(3) 156-170.
- Choi JS, Koren G, Nulman I (2013) Pregnancy and isotretinoin therapy. CMAJ 185(5): 411-413.
- Zasada M, Budzisz E (2019) Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol 36(4): 392-397.
- American Society of Health-System Pharmacists (2016) Vitamin A; The American society of health-system pharmacists.
- British national formulary (2015): BNF 69 (69 ed.). British Medical Association. pp. 701.
- Squires VR (2011) The role of food, agriculture, forestry and fisheries in human nutrition. EOLSS Publication (4): 121.
- Dewick PM (2009) Medicinal natural products.
- Leskov, Klenchin, Handy, Whitlock, Govardovskii, et al. (2000) The gain of rod phototransduction: reconciliation of biochemical and electrophysiological measurements. Neuron 27(3): 525-537.
- Ebrey T, Koutalos Y (2001) Vertebrate photoreceptors. Prog Retin Eye Res 20(1): 49-94.
- Hsu, Yi-Te, Molday Robert S (1993) Modulation of the CGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361(6407): 76-79.
- Arshavsky, Vadim Y, Lamb, Trevor D, Pugh, et al. (2002) G Proteins and Phototransduction. Annual Review of Physiology 64 (1): 153-187.
- Buss NE, Tembe EA, Prendergast BD, Renwick AG, George CF (1994) The teratogenic metabolites of vitamin A in women following supplements and liver. Hum Exp Toxicol 13(1): 33-43.
- D’Ambrosio DN, Clugston RD, Blaner WS (2011) Vitamin A metabolism: an update. Nutrients 3(1): 63-103.
- American Society of Health-System Pharmacists (2021) Tazarotene monograph for professionals. American society of health-system pharmacists.
- Wu L, Wu J, Zhou K, Cheng F, Chen Y (2011) Determination of isotretinoin in human plasma by high-performance liquid chromatographyelectrospray ionization mass spectrometry. J Pharm Biomed Anal 56(2): 324-329.
- Green AS, Fascetti AJ (2016) Meeting the vitamin A requirement: the efficacy and importance of β-carotene in animal species. Scientific World Journal 7393620.
- Von LJ, Vogt K (2000) Filling the gap in vitamin a research: molecular identification of an enzyme cleaving β-carotene to retinal. Journal of Biological Chemistry 275 (16): 11915-11920.
- Woggon, Wolf D (2002) Oxidative cleavage of carotenoids catalyzed by enzyme models and β-carotene 15,15’-monooxygenase. Pure and Applied Chemistry 74 (8): 1397-1408.
- Barar J, Asadi M, Mortazavi Tabatabaei SA, Omidi Y (2009) Ocular Drug Delivery, Impact of in vitro Cell Culture Models. Journal of ophthalmic & vision research 4(4): 238-252.
- Gaudana R, Ananthula HK, Parenky A, Mitra AK (2010) Ocular drug delivery. AAPS J 12(3): 348-360.
- Agrahari V, Mandal A, Agrahari V, Trinh HM, Joseph M, et al. (2016) A comprehensive insight into ocular pharmacokinetics. Drug Delivery and Translational Research 6(6): 735-754.
- Fenaux P, Wang ZZ, Degos L (2007) Treatment of acute promyelocytic leukemia by retinoids. Curr Top Microbiol Immunol 313: 101-128.
- Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134(6): 921-931.
- Moutier E, Ye T, Choukrallah MA, Urban S, Osz J, et al. (2012) Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. The Journal of biological chemistry 287(31): 26328-26341.
- Venkatesh K, Srikanth L, Vengamma B, Chandrasekhar C, Sanjeevkumar A (2013). In vitro differentiation of cultured human CD34+ cells into astrocytes. Neurology India. 61(4): 383-388.
- Wingender E (1993) Steroid/Thyroid hormone receptors. Gene Regulation in Eukaryotes. New York, USA, p.316.
- Molotkov A, Ghyselinck NB, Chambon P, Duester G (2004) Opposing actions of cellular retinol-binding protein and alcohol dehydrogenase control the balance between retinol storage and degradation. The Biochemical Journal 383(2): 295-302.
- Moore T, Holmes PD (1971) The production of experimental vitamin A deficiency in rats and mice. Laboratory Animals 5(2): 239-250.
- Van Pelt AM, de Rooij DG (1991) Retinoic acid can reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology. 128 (2): 697-704.
- Kean S (2012) Contraception research. Reinventing the pill: male birth control. Science 338 (6105): 318-20.
- US National Research Council (1995) National Research Council (US) Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995. Nutrient Requirements of the Laboratory Rat, National Academies Press, Washington (DC), USA.
- Teresa M, Cabezuelo, Rosa Zaragozá, Teresa Barber, Juan R, et al. (2020) Role of vitamin A in mammary gland Development and lactation. Nutrients 12 (80): 1-17.
- Cunningham TJ, Duester G (2015) Mechanisms of retinoic acid signaling and its roles in organ and limb development. Nat Rev Mol Cell Biol 16(2): 110-123.
- Layton A (2009) The use of isotretinoin in acne. Dermato-Endocrinology 1(3): 162-169.
- LiverTox (2020) Clinical and research information on drug-induced liver injury. Bethesda (MD): National institute of diabetes and digestive and kidney diseases 2012. Isotretinoin.
- Alliance Pharmaceuticals (2017) Isotretinoin 20mg capsules.
- European Medicines Agency (2017) Isotretinoin (oral formulations): CMDH scientific conclusions-Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s).
- Wysowski DK, Pitts M, Beitz J (2001) An analysis of reports of depression and suicide in patients treated with isotretinoin. Journal of the American Academy of Dermatology 45(4): 515-519.
- Ludot M, Mouchabac S, Ferreri F (2015) Inter-relationships between isotretinoin treatment and psychiatric disorders: Depression, bipolar disorder, anxiety, psychosis and suicide risks. World Journal of Psychiatry 5(2): 222-227.
- Brelsford M, Beute TC (2008) Preventing and managing the side effects of isotretinoin. Seminars in Cutaneous Medicine and Surgery 27(3): 197-206.
- Lambert RW, Smith RE (1989) Effects of 13-cis-retinoic acid on the hamster meibomian gland. The Journal of Investigative Dermatology 92(3): 321-325.
- Kremer I, Gaton DD, David M, Gaton E, Shapiro A (1994) Toxic effects of systemic retinoids on meibomian glands. Ophthalmic Research 26(2): 124-128.
- Sakai Y, Crandall JE, Brodsky J, McCaffery P (2004) 13-cis Retinoic acid (accutane) suppresses hippocampal cell survival in mice. Annals of the New York Academy of Sciences 1021(1): 436-440.
- Griffin JN, Pinali D, Olds K, Lu N, Appleby L, et al. (2010) 13-Cis-retinoic acid decreases hypothalamic cell number in vitro. Neurosci Res 68(3): 185-190.
- Nelson AM, Cong Z, Gilliland KL, Thiboutot DM (2011) TRAIL contributes to the apoptotic effect of 13-cis retinoic acid in human sebaceous gland cells. The British Journal of Dermatology 165(3): 526-33.
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, et al. (2008) Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. The Journal of Clinical Investigation 118(4): 1468-78.
- Wachter k (2009) Isotretinoin’s mechanism of action explored. Skin Allergy News. 40 (11): 32.
- Pendino F, Flexor M, Delhommeau F, Buet D, Lanotte M, et al. (2001) Retinoids down-regulate telomerase and telomere length in a pathway distinct from leukemia cell differentiation. Proceedings of the National Academy of Sciences of the United States of America 98 (12): 6662-6667.
- Bremner JD, Shearer KD, McCaffery PJ (2012) Retinoic acid and affective disorders: the evidence for an association. The Journal of Clinical Psychiatry 73(1): 37-50.
- Wysowski DK, Swartz L (2005) Relationship between headache and depression in users of isotretinoin. Archives of Dermatology 141(5): 640-641.
- Kontaxakis VP, Skourides D, Ferentinos P, Havaki-Kontaxaki BJ, Papadimitriou GN (2009) Isotretinoin and psychopathology: a review. Annals of General Psychiatry 8: 2.
- Borovaya A, Olisova O, Ruzicka T, Sárdy M (2013) Does isotretinoin therapy for acne cure or cause depression?. International Journal of Dermatology 52 (9): 1040-1052.
- FDA (2014). FDA information, side effects, and uses / Accutane (isotretinoin). U.S. Food and Drug Administration (FDA).
- Brazzell RK, Colburn WA (1982) Pharmacokinetics of the retinoids isotretinoin and etretinate: A comparative review. J Am Acad Dermatol 6(4): 643-651.
- FDA (2019) Drug Approval Package: Aklief. US Food and Drug Administration.
- Ruzicka T, Larsen FG, Galewicz D, Horváth A, Coenraads PJ, et al. (2004) Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: Results of a randomized, double-blind, placebo-controlled, multicenter trial. Archives of Dermatology 140(12): 1453-1459.
- Dawson MI, Xia Z (2012) The retinoid X receptors and their ligands. Biochim Biophys Acta 1821(1): 21-56.
- Nelson CH, Buttrick BR, Isoherranen N (2013) Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics. Current topics in medicinal chemistry 13(12): 1402- 1428.
- Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT (2003) CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. British journal of clinical pharmacology 56(3): 305-314.
- Panretin (2012) Alitretinoin gel, Daily Med. Eisai Inc.
- Khalil S, Bardawil T, Stephan C, Darwiche N, Abbas O, et al. (2017) Retinoids: a journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J Dermatolog Treat 28(8): 684-696.
- Kaplan G, Haettich B (1991) Rheumatological symptoms due to retinoids. Baillieres Clin Rheumatol 5(1): 77-97.
- Vahlquist A, Rollman O, Pihl Lundin I (1986) Tissue distribution of aromatic retinoid (etretinate) in three autopsy cases: drug accumulation in adrenals and fat. Acta Derm Venereol 66(5): 431-434.
- Mutschler E, Schäfer KM (2001) Arzneimittelwirkungen (8th Edn) Stuttgart: Wissenschaftliche Verlagsgesellschaft. p.728f.
- Happle R, Traupe H, Bounameaux Y, Fisch T (1984) Teratogenic effects of etretinate in humans. Dtsch Med Wochenschr 109(39): 1476-80.
- Halkier Sørensen L, Laurberg G, Andresen J (1987) Bone changes in children on long-term treatment with etretinate. J Am Acad Dermatol 16(5): 999-1006.
- Becker CD, Stichtenoth DO, Wichmann MG, Schaefer C, Szinicz L (2009) Blood Donors on Medication - an Approach to Minimize Drug Burden for Recipients of Blood Products and to Limit Deferral of Donors. Transfusion medicine and hemotherapy 36(2): 107-113.
- Qureshi ZP, Seoane VE, Rodriguez MR, Stevenson KB, Szeinbach SL (2011) Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiology and Drug Safety 20(7): 772-777.
- Geiger JM, Baudin M, Saurat JH (1994) The teratogenic risk with etretinate and acitretin treatment. Dermatology 189(2): 109-116.
- Zito PM, Mazzoni T (2021) Acitretin. StatPearls Treasure Island: StatPearls Publishing, Florida, USA.
- Rendon A, Schäkel K (2019) Psoriasis pathogenesis and treatment. International Journal of Molecular Sciences 20(6): 1475.
- Pilkington T, Brogden RN (1992) Acitretin: A review of its pharmacology and therapeutic use. Drugs 43(4): 597-627.
- WEBMD (2005) ACITRETIN Side Effects by Likelihood and Severity.
- Almond-Roesler B, Orfanos CE (1996) Trans-acitretin is metabolized back to etretinate. Importance for oral retinoid therapy. Hautarzt German 47(3): 173-177.
- Kolli SS, Pecone D, Pona A, Cline A, Feldman SR (2019) Topical Retinoids in Acne Vulgaris: A Systematic Review. Am J Clin Dermatol 20(3): 345- 365.
- American Society of Health-System Pharmacists (2014) AHFS DI Essentials™. ©Copyright 2022, Selected Revisions January 22, 2014. American society of health-system pharmacists, Inc., 4500 East-West Highway, Suite 900, Bethesda, Maryland, USA.
- Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, et al. (2006) Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clinical Interventions in Aging 1(4): 327-348.
- Duvic M, Nagpal S, Asano AT, Chandraratna RA (1997) Molecular mechanisms of tazarotene action in psoriasis. J Am Acad Dermatol 37(2 Pt 3): S18-S24.
- Heath MS, Sahni DR, Curry ZA, Feldman SR (2018) Pharmacokinetics of tazarotene and acitretin in psoriasis. Expert Opin Drug Metab Toxicol 14(9): 919-927.
- Qu L, Tang X (2010) Bexarotene: A promising anticancer agent. Cancer Chemotherapy and Pharmacology 65 (2): 201-205.
- Foster RH, Brogden RN, Benfield P (1998) Tazarotene. Drugs 55 (5): 705-711.
- Marks R (1998) Pharmacokinetics and safety review of tazarotene. J Am Acad Dermatol 39(4): 134-138.
- Menter A (2000) Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol 43(2): 31-35.
- Stefanaki C, Stratigos A, Katsambas A (2005) Topical retinoids in the treatment of photoaging. J Cosmet Dermatol 4(2): 130-134.
- Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, et al. (2007) Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 143(5): 606-612.
- Mayo Clinic (2017) Psoriasis-Diagnosis and treatment-Mayo Clinic.
- Gniadecki R, Assaf C, Bagot M, Dummer R, Duvic M, et al. (2007) The optimal use of bexarotene in cutaneous T-cell lymphoma. Br J Der 157(3): 433-440.
- Yuan S, Chan Jasper FW, Chik Kenn KH, Chan Chris CY, Tsang Jessica OL, et al. (2020) Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system. Pharmacological Research 159: 104960.
- Dragnev KH, Petty WJ, Shah SJ, Lewis LD, Black CC, et al. (2007) A proof of principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res 13(6): 1794-1800.
- Esteva FJ, Glaspy J, Baidas S, Laufman L, Hutchins L, et al. (2003) Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer. J Clin Oncol 21(6): 999-1006.
- Brunton L, Chabner B, Knollman B (2010) Goodman and Gilman’s the pharmacological basis of Therapeutics, 12th edn, McGraw-Hill Professional, New York, USA.
- Shahid MA, Ashraf MA, Sharma S (2021) Physiology, thyroid hormone. In: StatPearls. Treasure Island, StatPearls Publishing, Florida, USA.
- Bibi Z (2008) Role of cytochrome P450 in drug interactions. Nutr Metab 5: 27.
- Borse SP, Singh DP, Nivsarkar M (2019) Understanding the relevance of herb-drug interaction studies with special focus on interplays: a prerequisite for integrative medicine. Porto biomedical journal, 4(2): e15.
- Rowe A (1997) Retinoid X receptors. The International Journal of Biochemistry & Cell Biology 29(2): 275-258.
- Rolewski SL (2003) Clinical review: topical retinoids. Dermatology Nursing. 15(5): 447-450.
- Tazarotene (2022c) PubChem Compound Summary for CID 5381, National Center for Biotechnology Information.
- Tolaymat L, Dearborn H, Zito PM (2022) Adapalene. In: StatPearls. Treasure Island, Florida, USA.
- Asai Y, Baibergenova A, Maha D, Shannon H, Peter H, et al. (2016) Management of acne: Canadian clinical practice guideline. Canadian Medical Association Journal 188(2): 118-126.
- Drugs and Lactation Database (LactMed) (2018) Bethesda (MD): National Library of Medicine (US); 2006- Adapalene.
- Wolf JE, Kaplan D, Kraus SJ, Loven KH, Rist T, et al. (2003) Efficacy and tolerability of combined topical treatment of acne vulgaris with adapalene and clindamycin: a multi-center, randomized, investigatorblinded study. Journal of the American Academy of Dermatology. 49 (3 Suppl): S211-S217.
- Jain Gaurav K, Ahmed Farhan J (2007) Adapalene pretreatment increases follicular penetration of clindamycin: In vitro and in vivo studies. Indian Journal of Dermatology, Venereology, and Leprology 73(5): 326-9.
- Piskin S, Uzunali E (2007) A review of the use of adapalene for the treatment of acne vulgaris. Therapeutics and Clinical Risk Management 3(4): 621-624.
- Tolaymat, Leila, Zito, Patrick M (2018) Adapalene StatPearls.
- Cosio T, Di Prete M, Gaziano R, Lanna C, Orlandi A, et al. (2021) Trifarotene: a current review and perspectives in dermatology. Biomedicines 9(3): 237.
- Scott LJ (2019) Trifarotene: First Approval. Drugs 79 (17): 1905-1909.
- Brumfiel CM, Patel MH, Bell KA, Cardis MA (2021) Assessing the safety and efficacy of trifarotene in the treatment of Acne Vulgaris. Therapeutics and clinical risk management 17: 755-763.
- Trifarotene for acne (2021) Australian prescriber 44(4): 140-141.
- Aubert J, Piwnica D, Bertino B, Blanchet RS, Carlavan I, et al. (2018) Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-γ agonist trifarotene. Br J Dermatol 179(2): 442-456.
- British Association of Dermatologists (2020) ACITRETIN, what are the aims of this leaflet?
- Institute of Medicine (2001) Vitamin A. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Food and Nutrition Board of the Institute of Medicine pp 82-161.
- Retinal (2022) PubChem Compound Summary for CID 638015, National Center for Biotechnology Information.
- Isotretinoin (2022b) PubChem Compound Summary for CID 5282379, National Center for Biotechnology Information.
- National Institute for Health and Clinical Excellence (NICE), (2009).
- Prepn (1992) Chandraratna RA, EP 284288; idem, U.S. Patent 5,089,509.
- Tang Liu DD, Matsumoto RM, Usansky JI (1999) Clinical pharmacokinetics and drug metabolism of tazarotene: a novel topical treatment for acne and psoriasis. Clin Pharmacokinet. 37(4): 273-287.
- Topical retinoids (2016) DermNet New Zealand.
- World Health Organization (2009) Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. Pp. 500.
- Flora XL, Patricia T, Torres LV, Jerry T, Hun SD, et al. (2018) Practical management of acne for clinicians: an international consensus from the global alliance to improve outcomes in acne. Journal of the American Academy of Dermatology 78(2): S1- S23.e1.
Article Type
Review Article
Publication history
Received Date: April 20, 2022
Published: July 11, 2022
Address for correspondence
Bunu J Samuel, Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Nigeria
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Bunu JS, Miediegha O, Agbolo T, Adugo M, Usifoh OC. Review of Vitamin A Structural Analogues and their Pharmacokinetic Parameters. 2022- 4(4) OAJBS.ID.000466.
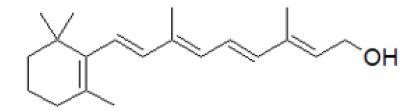
Figure 1: Retinol: (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl) nona-2,4,6,8-tetraen-1-ol.
Figure 2: β-carotene.
Figure 3: Visual cycle (Ebrey, & Koutalos, 2001).
Figure 4: Retinal, (retinaldehyde): (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl) nona-2,4,6,8 tetraenal.
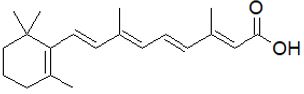
Figure 5: Retinoic acid (tretinoin): (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl) nona-2,4,6,8-tetra- 1-enoic acid.
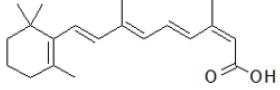
Figure 6: Isotretinoin: (2Z,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetra-1-enoic acid.
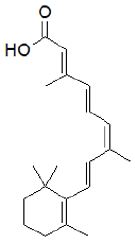
Figure 7: Alitretinoin, ((2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl) nona-2,4,6,8-tetra-1-enoic acid).
Figure 8: Etretinate.
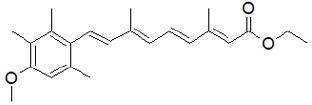
Figure 9: Acitretin.
Figure 10: Tazarotene: ethyl 6-[(4,4-dimethyl-3,4-dihydro-2H-thiochromen-6-yl)ethynyl]pyridine-3-carboxylate.
Figure 11: Bexarotene.
Figure 12: Adapalene.
Figure 13: Trifarotene.