Prevalence and Multidrug Resistant Patterns of Candida sp. isolated from Selected Rural Diabetics and Non-Diabetic Populations, Sirajganj, Bangladesh
ABSTRACT
Oral candidiasis is a type of fungal infection that mostly affects the oral mucosa. Candida sp., the primary causative agent, is a highly adaptable commensal organism that is well adapted to its host. Contemporary knowledge of pathogens and their antibiotic susceptibility patterns is critical for keeping antibiotic policies up to date. This study was undertaken by collecting and analyzing samples from rural areas of Sirajganj, Bangladesh to determine the prevalence of Candida species that cause oral candidiasis and to determine their susceptibility pattern to antifungal drugs. SPSS analysis was done for quantitative data, i.e., the positive sample numbers and the different ages, sex, and medical conditions of carriers. A total of 304 clinical samples were collected aseptically from patients with oral candidiasis, with 64 showing positive growth with Candida sp. Further analysis revealed that the oral prevalence of Candida sp. among diabetic people was higher than that of non-diabetics, where Candida albicans (80.8%) was the most prevalent species, followed by Candida glabrata (9.6%) and Candida krusei (9.6%). We also found that Candida sp. grow best in acidic conditions (pH level 7 or less). Finally, varying levels of susceptibility to antifungal drugs were also evaluated. Nystatin and Amphotericin B had the highest sensitivity for Candida glabrata, each at 100.0%, and Fluconazole had 100% sensitivity for Candida krusei. Itraconazole and Voriconazole were highly resistant to all examined isolates (50-62%) except for Itraconazole, which exhibited 80% susceptibility to Candida krusei. These findings are crucial to identifying causative pathogens and determining the sensitivity patterns of drugs, as well as improving clinicians’ knowledge of how to select the best medicine and, as a result, contributing to patient diagnosis and treatment.
KEYWORDS
Oral candidiasis; Diabetics and Non-diabetic populations; Candida. isolates; Antifungal drugs
INTRODUCTION
Oral candidiasis, a form of candidiasis, is the most frequent mucocutaneous mycosis of the oral cavity. It is caused by the genus Candida species which can be existed in the oral cavity of 53% of the general population and is usually encountered as an opportunistic commensal organism [1,2]. The transformation of a commensal organism into a pathogen is influenced by a number of predisposing factors that alter the oral cavity’s microenvironment and favor the emergence of opportunistic infections. For diabetic patients, in addition to the oral microenviron¬ment-modifying factors (poorly fitting dentures, chronic antiseptic use, prolonged dummy use in children, poor oral hygiene, pH, smoking, and alcoholism), there are additional risk factors such as increased salivary glucose level [3]. Long-term administration of broad -spectrum antibiotics, extremes of age, drug treatment, pregnancy, occupation, education, etc. can also affect the severity of infection [4]. On the other hand, can lead to local discomfort, altered taste sensations, and dysphagia due to oesophageal overgrowth, resulting in poor nutrition, delayed recovery, and a long stay in the hospital [5]. This can cause serious infection in diabetic people, resulting in considerable morbidity and mortality. A mortality rate of 71%to 79% is associated with systemic candidiasis [6]. The species of Candida isolated in the oral cavity are C. albicans, C. tropicalis, C. parapsilosis, C. krusei, C. guillermondii, C. glabrata and C. dubliniensis [7]. Despite the foregoing data, the frequency of oral candidiasis is unknown, as these infections often go undetected by the clinician. The Candida species identification is critical because non-albicans Candida (NAC) species are also major pathogens and are resistant to antifungal treatments [8]. Nonetheless, the disease’s diagnosis is critical, as it could be the first sign of a systemic disorder, including diabetes [9]. Despite the fact that antifungal pharmaceutical treatments are determined by trial and error, in vitro susceptibility testing is required for directing or changing the treatment. Antifungal susceptibility testing will ideally become more useful in clinical antifungal therapy guidance as procedures and applications improve [10].
The present study was undertaken to determine the prevalence of Candida sp. causing oral candidiasis and figure out the current susceptibility pattern to antifungal drugs and to evaluate several predisposing factors. This will enable clinicians to facilitate the effective treatment and management of patients with symptoms of oral candidiasis.
MATERIALS AND METHODS
Ethical Consideration
It was also approved by the institutional ethical grant committee of Khwaja Yunus Ali University (KYAU), Enayetpur, Sirajganj, Bangladesh.
Study Population and Biological Samples
The consent of the patients and the control group was made in writing through the TIC (Term of informed consent). The signature was also taken from each patient before his/her entry into the study, with the understanding that the collected data will be used for non-commercial research purposes and that names will be kept confidential. The assessment of participants was conducted by a brief interview and completion of a questionnaire that contained socio-economic status (age, marital status, and occupation), dental status, smoking habits, and duration of diabetes mellitus. The final selection was based on clinical examination of whether they have signs or symptoms of oral candidiasis or not. A healthy control group (neither diabetic nor smoker) comprised of healthy volunteers matched for age, sex, dental status, and smoking habits was also maintained who did not show any alterations in the oral cavity. These selected patients were free of any type of disease.
Study Area, Patient Selection and Sample Size
The cross-sectional study was carried out between December 2020 and August 2021. Samples were collected from Enayetpur, Sirajganj, Bangladesh, including Gopalpur, Ghoprekhi, Khukni, Mondol Para, Betil & Uttor Para. Primarily 500 Diabetic patients, both smokers and non-smokers, were selected. Among them, 304 patients were finally enrolled in the study based on the information from the questionnaire and clinical manifestations. All of them were not on antibiotic or corticosteroid therapy during the previous 4 weeks while all cases were receiving treatment for their diabetes mellitus.
Sample Collection and Processing
Oral swabs: Oral swab was collected from patches of white exudates in place on the mucous membrane of the mouth using a sterile cotton wool swab taking care not to contaminate the swab with saliva and was returned to its sterile container.
Blood samples: Lancing device was used on the side of the fingertip to get a drop of blood. Then Blood glucose was measured by glucometer using a strip (BioHermes, Limpid, China; Range: 7.2-above). Fasting blood glucose was also measured in all participants.
Urine sample: Urine sample was collected from all participants using a sterile container for the corresponding sugar measurement. After that, the amount of glucose and protein label was determined using a standard urine test strip (which may comprise up to 2 different chemical pads or reagents) by immersing it in the urine sample and observing the change of color.
Saliva sample: A Saliva sample from all participants for detection of pH was collected into a sterile container and pH was measured using a pH strip by observing the color when dipped into the sample container. The color was matched with the indicator chart in the pH test strips’ packaging.
Isolation and Identification of Bacteria
Sabouraud dextrose agar (SDA) was used to identify the pathogens. Media were prepared according to the manufacturer’s guidelines and sterilized at 121°C for 15 minutes at 15 lb Psi. Aseptically collected oral samples were cultured by spreading the sample onto sterile SDA. Then the petri plates were incubated at 37ºC for 24-48 hours. After incubation, the plates which did not show any evidence of growth after that particular period were considered negative and discarded. Subsequent streaking was performed with a sterile inoculation loop to obtain a pure colony [11].
Microscopic Examination, Morphology Studies, And Preservation of Candida Sp.
All Candida sp. isolates were identified through cultural, morphological, and microscopic tests following standard procedures. The morphology of Candida spp. including the surface appearance, margin forms, and elevation were observed on SDA. Further subculture on HiCrome™ Candida Differential Agar (CDA) was also conducted to identify the species-specific colonies. One single colony from each SDA plate was streaked on a clean glass slide, stained with Gram-stain, and examined microscopically under an oil immersion lens. Then they were kept at 4°C for further experiments while 15% glycerol was used for long-term storage [12].
Antifungal Susceptibility Testing
Susceptibility to Fluconazole, Nystatin, Amphotericin B, Itraconazole, and Voriconazole was performed by direct disk diffusion methods. For standard disk diffusion testing, inoculums were prepared by picking 4-5 colonies from 24-hour cultures on SDA. Colonies were suspended in 5 mL of sterile saline and turbidity was adjusted to 0.5 McFarland. The final concentrations of the inoculums were 1-5×106 CFU/mL. A sterile cotton swab was dipped into the suspension and excess fluid was removed. The inoculum was spread by evenly streaking the swab onto the surface of Mueller-Hinton agar. After applying antifungal disks, the plate was incubated at 37 °C for 24-48 hours and the inhibition diameters around the disks were measured and recorded [13].
Data Presentation, Interpretation and Analysis of Findings
Quantitative data was entered in SPSS files and calculated manually. Calculations the positive sample numbers, and distribution based on the different age, sex, and medical condition of carriers were accomplished.
RESULTS
A total of 304 diabetic patients were selected for this study. The diabetic status of the patients was confirmed by measuring glucose levels using strips. Laboratory work was carried out for 9 months in the Department of Microbiology at Khwaja Yunus Ali University in Sirajganj. The findings of the study were as follows and shown in the form of tables, figures, and texts.
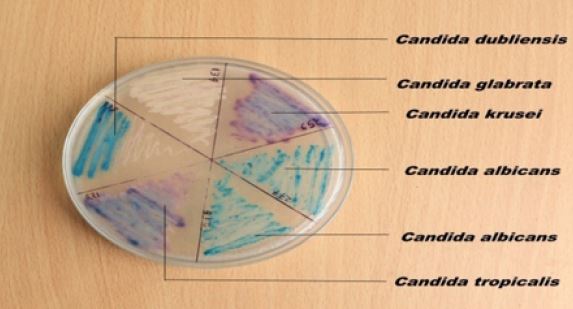
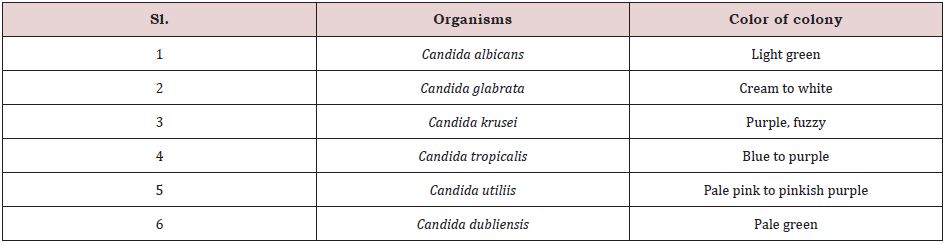
Colonial morphology: Candida species appeared as dry, creamy, spherical surfaces with a white to cream color when seen on SDA agar. Subculture on CDA agar to identify individual Candida species revealed the following distinct color of Candida sp. (Table 1).
Cell morphology: Following Gram staining, observation of slides under 100X magnification using a light microscope revealed that some Candida cells exhibit pseudo hyphae. Blastoconidia were present, and they were oval to round to elongated in shape with vermiculate branches.
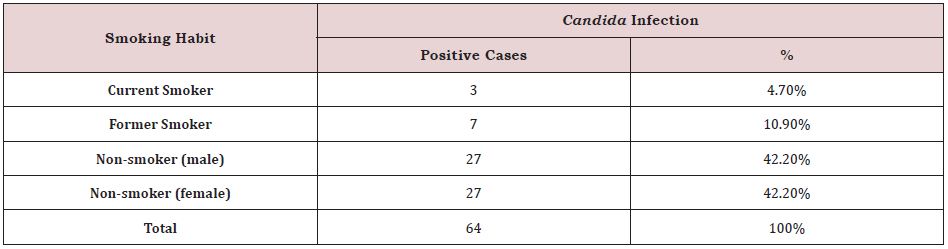
Prevalence of Oral Candida Infection on Smoking Habits in the Total Population:
Out of 304 patients, 64 were Candida positive, with non-smoker male patients having the highest prevalence of oral Candida infection with 27 cases (42.2%) and non-smoker female patients having 27 cases (42.2%). This data also shows that out of 64 Candida positive cases, 7 cases (10.9 %) were caused by previous smoking, followed by 3 instances caused by current smoking (4.7%) (Table.2).
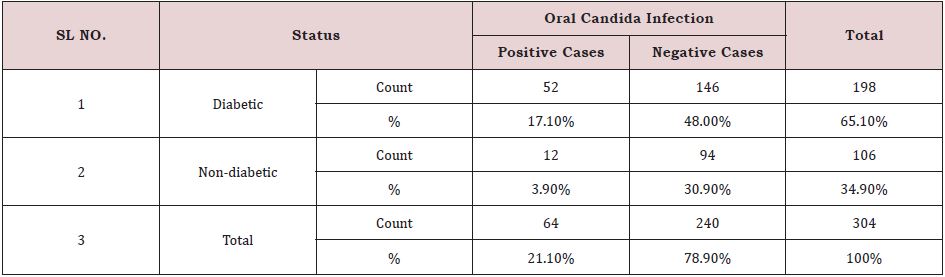
Out of 304 individuals, 64 cases were Candida positive, accounting for 21.1% of the total cases. The remaining 240 patients (78.8%) were Candida negative. There were 198 diabetics and 106 non-diabetics among the participants. Diabetic persons (52 cases) had the highest frequency of oral Candida infection (17.1%) compared to non-diabetic individuals (3.9%) (Table. 3).
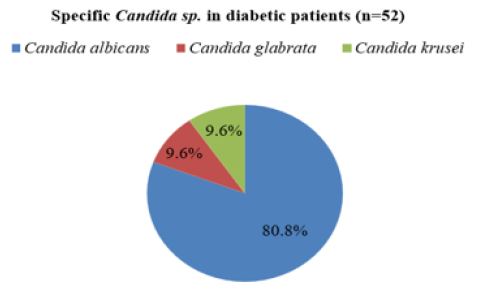
Prevalence of oral Candida infection by specific Candida sp. in diabetic patients: Among 198 diabetic patients, Candida was not identified in 146 patients and isolated from 52 diabetic patients. Among them, C.albicans was found in 42 cases with 80.8% followed by C.glabrata in 05 cases with 9.6% and C.krusei in 5 cases with 9.6%. C.albicans was the most predominant species in diabetic patients (Figure 1,2).
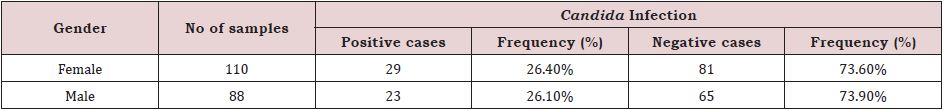
Prevalence of Oral Candida Infection by Gender Distribution In Diabetic Patients
It is clearly indicated from the table that oral Candida infection was more common in diabetic women than in diabetic men. Females accounted for 110 (26.4%) of the 198 diabetic cases, while males accounted for 88 (26.1%) of the cases. Candida was not found in 73.6% of women and 73.9%of men (Table 4).
Prevalence Of Oral Candida Infection with Age Distribution in The Diabetic Patient
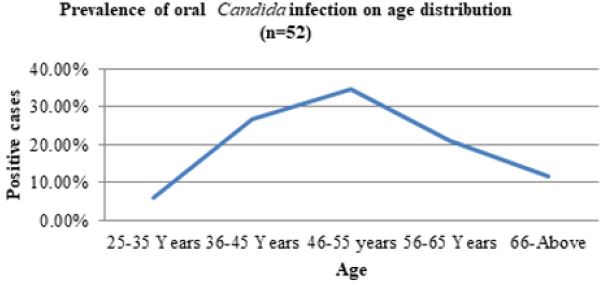
Based on 52 Candida infected cases, the most common age group affected by Candida sp. in diabetes patients was 46-55 years, with 18 cases (34.6%), followed by 36-45 years with 14 cases (26.9%), 56-65 years with 11 cases (21.2%) and 66-above years with 6 cases (11.5%). The age range of 25-35 years was the least impacted, with only three instances (5.8%). With 34.6%, the age group 46-55 years old had the highest frequency of oral Candida infection in diabetes patients (Figure 3).
Prevalence Of Oral Candida Infection on Education in Diabetic Patients
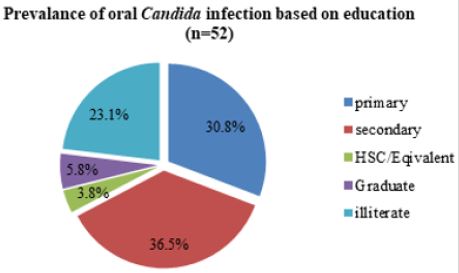
Graph (Fig. 6) shows that, out of 52 Candida infected diabetic cases, the most prevalent impacted category was secondary, with 19 instances (36.5%), followed by primary with 16 cases (30.8%), and illiteracy with 12 cases (23.1%). The graduate group with 3 cases (5.8%) and the HSC/Equivalent group with 2 cases (3.8%) were the least affected. In diabetes individuals, educated people are less prone to oral Candida infection (Figure 4).
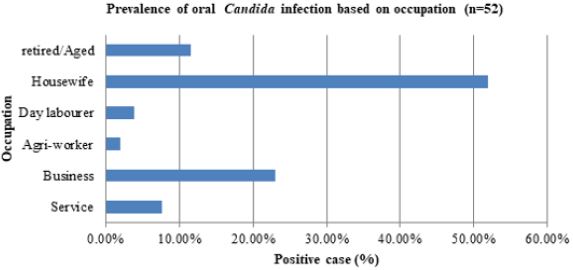
Prevalence of Oral Candida Infection on Occupation In Diabetic Patients
The most prevalent impacted category, with 27 instances (51.9%), was the housewife, followed by the business group with 12 cases (23.1%), the aged/retired group with 6 cases (11.6%), and the service group with 4 cases (7.7%). The least afflicted groups were agro-workers, who had one case (1.9%) and day laborers, who had two instances (3.9%) (Figure 5).
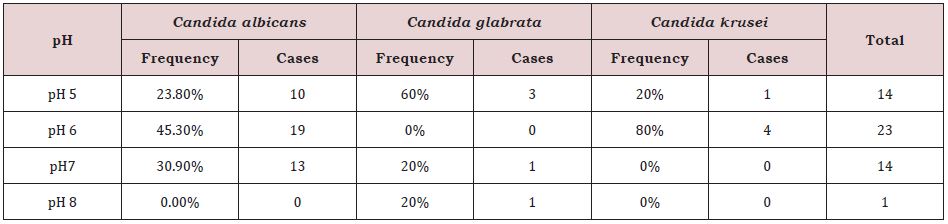
Prevalence of Candida Sp. On The pH Level of Saliva in Diabetic Patients
C. albicans was found at the highest frequency in the pH 6 group, with 19 cases (45.3%), followed by the pH 7 group with 13 cases (30.9%), and the pH 5 group with 10 cases (23.8%) while absent in the pH 8 group.In the case of C. glabrata, the highest frequency was seen in pH 5 group with 3 cases (60%) followed by pH 7 group with 1 case (20%) and pH 8 group with 1 cases (20%). C. glabrata was absent in pH 6 group. For C. krusei, the highest frequency of was observed in the pH 6 group with 4 cases (80%) followed by the pH 5 group with 1 cases (20%). C. krusei was absent in pH 7 and pH8 groups (Table 5).
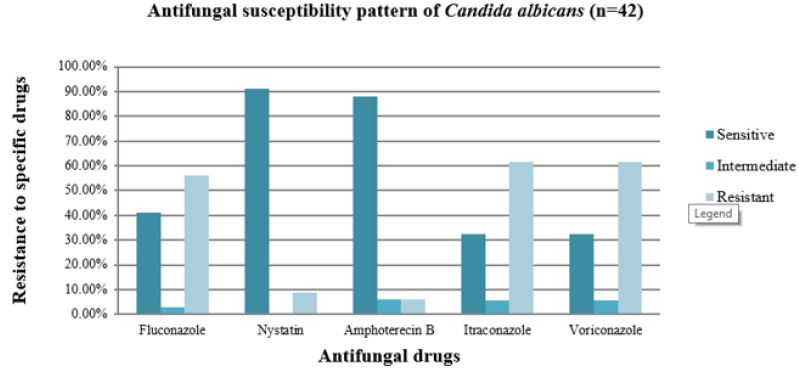
Antifungal Sensitivity Pattern of C. Albicans
Nystatin was most susceptible to C.albicans (91.2%), followed by Amphotericin B (88.2%), Fluconazole (41.2%), Itraconazole (32.4%), and Voriconazole (32.4%). Data also indicates that Itraconazole and Voriconazole were the most resistant to C.albicans with each 61.8%.The most effective antifungal medicines against C.albicans were Nystatin and Amphotericin B, as seen in this diagram. Voriconazole and Itraconazole were similarly found to be less efficient antifungal medicines against C.albicans (Figure 6).
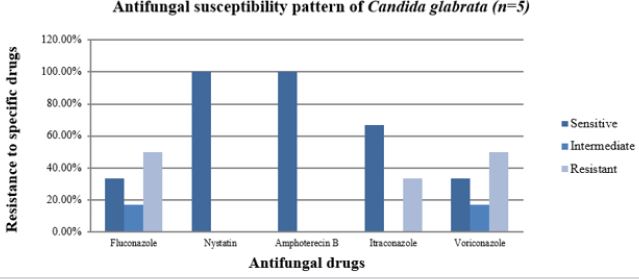
Antifungal Sensitivity Pattern of C.glabrata
Figure 7 shows that Nystatin and Amphotericin B were most sensitive to C.glabrata with each 100.0% followed by Itraconazole with 66.7%, Fluconazole with 33.3% and Voriconazole with 33.3%respectively. Data also indicates that, Fluconazole and Voriconazole were most resistant to C. glabrata with each 50.0%. Fluconazole and Voriconazole were intermediate to C.glabrata with each 16.7%. This diagram shows that Nystatin and Amphotericin B were the most effective antifungal drugs against C. glabrata. It also indicates that Voriconazole and Fluconazole were less effective antifungal drugs against C.glabrata.
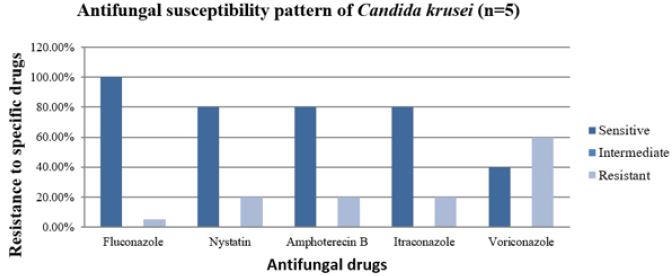
Antifungal Sensitivity Pattern of C.krusei
Data obtained from antifungal sensitivity tests show that Fluconazole was most sensitive to C. krusei with 100% followed by Itraconazole, Nystatin and Amphotericin B with each 80% respectively. This table also indicates that, Voriconazole was the most resistant to C. krusei with each 60 %. All these five antifungal drugs did not show intermediate sensitivity to C. krusei. This diagram shows that, Fluconazole was most effective antifungal drug against C. krusei. Nystatin and Amphotericin B were also effective antifungal drugs against C. krusei. It also indicates that Voriconazole was least effective antifungal drug against C. glabrata (Figure 8).
DISCUSSION
Infections by Candida sp. have become more common in recent years and the most prevalent opportunistic infection among diabetic patients is oral candidiasis, which poses a significant treatment challenge. As a result, it is recommended to figure out the species involved in the infection, and to determine their susceptibility to antifungal drugs. This study revealed that non-smoker male patients and non-smoker female patients had the highest and same prevalence of oral Candida infection (42.2%). Previous smokers and current smokers were also infected at a rate of 10.9% and 4.7%, respectively. Statistically, there was no association between oral Candida infection and cigarette smoking. Abu-Elteen et al. (2006) reported that the frequency of Candida isolation was significantly higher in smokers than in non-smokers in both diabetics and controls, which did not match with this study [14].
In this study, the oral prevalence of Candida species among diabetic people (17.1%) was higher than those of non- diabetics (3.9%) which are consistent with numerous previous studies, which have shown that diabetes mellitus is a major predisposing factor to symptomatic candidiasis, oral or otherwise. Sampath et al., (2019) found similar output that the population density of yeasts in diabetics was significantly higher than that of controls implying that the oral mucosa in diabetics offers a less hostile ecosystem for oral Candida colonization [15]. Local variables such as xerostomia and denture use may be to blame, as well as systemic issues such as glycemic management and treatment regimens. The persistence of aciduric yeasts in the oral cavity has been linked to a sugar-rich oral environment in uncontrolled diabetes due to high salivary glucose levels [16]. Dietary carbohydrates are also known to enhance yeast adhesion, biofilm development, and colonization in the oral environment, which could be another contributing factor [17].
C. albicans (80.8%) was the most prevalent species in diabetic patients followed by C.glabrata (9.6%) and C. krusei (9.6%). These values are similar to those reported in the literature, which describes this species as the most prevalent Candida species in the oral cavity of diabetic patients, with values between 67 and 98% [18,19]. Females have a higher risk of Candida colonization (26.4%) than males, according to the current study (26.1%). Oral mucosal inflammation in females related to periodontitis has been suggested by several researchers as a possible cause of yeast infestation in females which is consistent with the findings of this investigation [20]. The increasing age is reported as an important risk factor for developing oral fungal infection in diabetic patients, which is agreed with this study [21]. In this study, the most common age group affected by Candida sp. in diabetic patients was 46-55 years (34.6%) followed by 36-45 years (26.9%), 56-65 years (21.2%) and 66-above years (11.5%).
In this study, we found that Candida species grow best in acidic condition (pH level 7 or less). Out of 52 Candida sp. isolated from diabetic patients, the highest frequency was seen in the pH 6 group with 23 cases followed by pH 5 and pH 7 groups with each 14 cases. This study confirms prior findings that an acidic pH in the mouth favors candidal growth over normal bacterial microbiota growth [22]. Three Candida species tested in this study, found to have varying levels of susceptibility to the antifungal drugs used. Nystatin and Amphotericin B had the highest sensitivity for C. glabrata, each at 100%, and Fluconazole had 100.0% sensitivity for C.krusei. Nystatin, also effective against C.albicans with a sensitivity of 91.2 %, was followed by Amphotericin B. (88.2 %). Itraconazole and Voriconazole were highly resistant to all examined isolates (50- 62%), except for Itraconazole, which exhibited 80 % susceptibility to Candida krusei. Overall, the results of the in vitro susceptibility testing were similar to those obtained in previous work [23].
CONCLUSION
Doctors need access to antifungal susceptibility patterns due to the increased rates of Candida infection and drug-resistant phenotypes to enhance treatment outcomes. Species identification of Candida is critical for rapid, inexpensive, and successful therapy since it aids in the optimal selection of the therapeutic drug. This is extremely valuable data on mouth Candida infections in diabetic patients chosen from rural areas, as well as research into antifungal activity for the treatment of oral candidiasis in diabetic patients. The results from this research will help the doctor to determine which drugs are likely to be most effective in treating diabetic oral Candia infections.
ACKNOWLEDGEMENT
We thank the authorities of Prochesta Model School, Enayetpur, Sirajganj, for their assistance in sample collection. Our heartfelt gratitude also goes to Md. Alamgir Hossain Akabar for providing us with sample collection opportunities at their Prochesta Model School and for guiding us through diverse and exciting projects. We thank our fellow labmates at Khwaja Yunus Ali University and University of Chittagong for the stimulating discussions. We would also like to thank our students, Arafin Sazzad, Labonno Ahmed, Shohan Islam, and Abu Musa Shekh. In particular, we are grateful to Mr. Mohitul Ameen Ahmed Mustafi for enlightening us with the first glimpse of research for data analysis.
REFERENCES
- Akpan A, Morgan R (2002) Oral candidiasis. Postgraduate Medical Journal 78(922): 455-459.
- Coronado CL, Jiménez SY (2013) Clinical and microbiological diagnosis of oral candidiasis. Journal of Clinical and Experimental Dentistry 5(5): e279.
- Vila T, Sultan AS, Montelongo JD, Jabra RMA (2020) Oral candidiasis: a disease of opportunity. Journal of Fungi 6(1): 15.
- Farah CS, Lynch N, McCullough MJ (2010) Oral fungal infections: an update for the general practitioner. Australian Dental Journal 55: 48-54.
- Nagao Y, Hashimoto K, Sata M (2012) Candidiasis and other oral mucosal lesions during and after interferon therapy for HCV-related chronic liver diseases. BMC Gastroenterology 12(1): 1-9.
- Patel M (2022) Oral cavity and candida albicans: colonisation to the development of infection. Pathogens 11(3): 335.
- Rodrigues CF, Rodrigues ME, Henriques M (2019) Candida sp. infections in patients with diabetes mellitus. Journal of Clinical Medicine 8(1): 76.
- Du J, Wang X, Luo H, Wang Y, Liu X et al. (2018) Epidemiological investigation of non albicans Candida sp. recovered from mycotic mastitis of cows in Yinchuan, Ningxia of China. BMC Veterinary Research 14(1): 1-9.
- Almadih A, Al ZM, Dabel S, Alkhalaf A, Al Mayyad A et al. (2018) Orthodontic treatment consideration in diabetic patients. Journal of Clinical Medicine Research 10(2): 77.
- Perfect JR, Ghannoum M (2020) Emerging issues in antifungal resistance. Infectious Disease Clinics 34(4): 921-943.
- Geethalakshmi V, Jasmine K, John AP, Prathap P (2021) Effectiveness of sabouraud’s dextrose agar and dermatophyte test medium in detection of candidiasis and dermatophytosis in superficial skin lesion. Journal of Clinical and Diagnostic Research 15(8).
- Kumari A, Mankotia S, Chaubey B, Luthra M, Singh R (2018) Role of biofilm morphology, matrix content and surface hydrophobicity in the biofilm-forming capacity of various candida sp. Journal of Medical Microbiology 67(6): 889-892.
- Mahboob N, Iqbal H, Ahmed M, Magnet MMH, Mamun KZ (2019) Disk diffusion method in enriched mueller hinton agar for determining susceptibility of candida isolates from various clinical specimens. Journal of Dhaka Medical College 28(1): 28-33.
- Abu EKH, Hamad MA, Salah SA (2006) Prevalence of oral candida infections in diabetic patients. Bahrain Med Bull 28(1): 1-8.
- Sampath A, Weerasekera M, Dilhari A, Gunasekara C, Bulugahapitiya U, et al. (2019) Type 2 diabetes mellitus and oral candida colonization: Analysis of risk factors in a Sri Lankan cohort. Acta Odontologica Scandinavica 77(7): 508-516.
- Samaranayake LP, MacFarlane TW (1985) On the role of dietary carbohydrates in the pathogenesis of oral candidosis. FEMS Microbiology Letters 27(1): 1-5.
- Santana IL, Gonçalves LM, Vasconcellos AAD, Silva WJ, Cury JA (2013) Dietary carbohydrates modulate Candida albicans biofilm development on the denture surface. PloS one 8(5): e64645.
- Aitken SJ, Lund RG, González J, Huenchunao R, Perez Vallespir (2018) Diversity, frequency and antifungal resistance of Candida sp. in patients with type 2 diabetes mellitus. Acta Odontologica Scandinavica 76(8): 580-586.
- Hammad MM, Darwazeh AM, Idrees MM (2013) The effect of glycemic control on Candida colonization of the tongue and the subgingival plaque in patients with type II diabetes and periodontitis. Oral Surgery Oral Medicine Oral Pathology and Oral Radiology 116(3): 321-326.
- 20. Kumar BV, Padshetty NS, Bai KY, Rao MS (2005) Prevalence of Candida in the oral cavity of diabetic subjects. The Journal of the Association of Physicians of India, 53, 599-602.
- Lamster IB, Lalla E, Borgnakke WS, Taylor (2008) The relationship between oral health and diabetes mellitus. The Journal of the American Dental Association 139: 19S-24S.
- Rautemaa R, Ramage G (2011) Oral candidosis clinical challenges of a biofilm disease. Critical Reviews in microbiology 37(4), 328-336.
- Khadka S, Sherchand JB, Pokhrel BM, Parajuli k, Mishra SK et al. (2017) Isolation, speciation and antifungal susceptibility testing of candida isolates from various clinical specimens at a tertiary care hospital, Nepal. BMC Research Notes 10(1): 1-5.
Article Type
Research Article
Publication history
Received Date: September 13, 2022
Published: November 07, 2022
Address for correspondence
Mohammad Zakerin Abedin, Department of Microbiology, Khwaja Yunus Ali University, Bangladesh
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Mohammad ZA, Mst. Maisha M, Farjana AK, Samia S, Samim M, Mst. Nadia A, et al. Prevalence and Multidrug Resistant Patterns of Candida sp. isolated from Selected Rural Diabetics and Non-Diabetic Populations, Sirajganj, Bangladesh. 2022- 4(6) OAJBS.ID.000512.
Figure 1: Colonial characteristics of Candida species on Candida differential chromogenic agar isolated from the oral cavity of a diabetic patient.
Figure 2: Prevalence of oral candida infection by specific Candida sp. in diabetic patients.
Figure 3: Prevalence of oral candida infection on age distribution in diabetic patient.
Figure 4: Prevalence of oral candida infection on education in diabetic patient.
Figure 5: Prevalence of oral Candida infection on occupation in diabetic patient.
Figure 6: Bar diagram showing the incidence of antifungal susceptibility pattern of C.albicans.
Figure 7: Bar diagram showing the incidence of antifungal susceptibility pattern of C. glabrata.
Figure 8: Bar diagram showing the incidence of antifungal susceptibility pattern of C. krusei.
Table 1: Cultural characteristics of Candida sp.
Table 2: Prevalence of oral Candida infection on smoking habit in total population.
Table 3: Prevalence of oral candida infection on diabetic status in total population (n=304).
Table 4: Prevalence of oral Candida infection on gender distribution in diabetic patient (n=198).
Table 5: Prevalence of Candida sp. on pH level of saliva in diabetic patients (n=52).