Practical Recommendations on the Management of Issues Related to the Levonorgestrel 52mg Intrauterine System: A Delphi Panel
ABSTRACT
Objective: To gather the routine practice of Brazilian physicians with large experience on the Levonorgestrel 52mg intrauterine
system (LNG IUS) use in order to generate practical recommendations on how to manage common aspects related to it.
Material and Methods: Twenty gynecologists with large experience with LNG IUS answered 39 questions grouped into five
main topics: exams before the procedure, technical aspects of the device placement, practical recommendations, irregular bleeding,
and pregnancy. A second round of questions was carried when responses were very divergent.
Results: Eighteen panelists responded to the questions. Recommendations that had more than 90% of agreement were i) the
gynecological exam is the most important assessment before the insertion of LNG IUS; ii) in case of expulsion, a new device could be
inserted; iii) there is no minimum age after menarche to use LNG IUS as a contraceptive, and iv) the device can be maintained after
menopause. All the panelists responded that the duration of the insertion procedure was less than 10 minutes. Some topics did not
reach more than 50% of agreement.
Conclusion: Most aspects related to LNG IUS usage reached an agreement in terms of clinical practices among physicians with
different backgrounds, and also tend to correlate to the available guidelines. However, despite the availability of LNG IUS in Brazil
for many years, there is still some disagreement regarding routine practices. There is a need for continuous education of physicians
and the standardization of certain procedures to expand the use of this contraceptive method.
KEYWORDS
Contraception; Hormonal Contraception; Levonorgestrel; Long-Acting Reversible Intrauterine Devices
INTRODUCTION
Long-acting reversible contraceptive (LARC) methods are those with at least 3 years of duration and without the need for active adherence once initiated, such as copper intrauterine device (Cu-IUD), the levonorgestrel 52mg intrauterine system (LNG IUS) and the etonogestrel-implant [1]. The LARCs have the lowest failure rates, equivalent to permanent contraception, high continuation rate, and rare adverse events in comparison with short-acting methods like the pill, vaginal ring, or patch [1-3]. These methods are recommended for all women who want effective contraception, including adolescents, nulligravidas, women in postpartum or post-abortion periods, and with comorbidities associated with contraindications to estrogen-containing methods [2].
The LNG IUS (Mirena®, Bayer Oy, Turku, Finland) is one of the most effective contraceptive methods with an annual failure rate of 0.1 [4] and has been available in several countries for 30 years and in Brazil since the year 2000. Besides the high contraceptive efficacy, the LNG IUS is also used as a treatment to reduce heavy menstrual bleeding, dysmenorrhea and endometriosis-associated pain (albeit this use is off-label) [2,4]. However, despite the high contraceptive efficacy, few side-effects, non-contraceptive benefits, and a large body of evidence in the scientific literature [5], it is still underutilized in many settings including adolescent girls and young women, a population with high risk of unplanned pregnancy [6]. There are several reasons for this underutilization: lack of training of healthcare providers in intrauterine device (IUD) placement, lack of reimbursement or incomplete insurance coverage, refusal by the women who do not have enough knowledge about the safety and efficacy, and the unavailability of the product in the majority of the Brazilian public health institutions [7,8].
Furthermore, one additional challenge is the scarce scientific literature addressing practical clinical issues [8-10]. As a result, many physicians may feel uncomfortable in managing routine aspects such as the interruption of the previous contraceptive method in use before LNG IUS placement, which exams are necessary before the procedure and how to manage insertion related pain, anxiety and occasional adverse events.
The Delphi method is a feasible tool to obtain opinions on a very specific subject from experts, prioritizing their experience and ensuring that the participants feel comfortable expressing their thoughts and practices. It is a qualitative survey method that allows the collection of opinions and experiences from experts in different geographical areas and makes it possible to deal with complex problems without face-to-face interaction [11]. This overcomes the need to conduct large face-to-face inquiries, which are time and resource consuming [12]. Important aspects of the Delphi method are the anonymity of the respondents, selection process, feedback from the experts after each round of questions, the timing for responses, and participation of at least 10 panelists [11]. Feedback and sharing the results with the panel are important because the panel can reconsider some responses after knowing the point of view of the other panelists.
Due to the above characteristics, we selected the Delphi method for our inquiry, and we present the results of a study conducted with Brazilian gynecologists with large experience in inserting and managing the use of LNG IUS at office level. This panel aimed to generate practical recommendations for the management of issues related to the use of LNG IUS.
METHODS
Twenty gynecologists with a large amount of experience in the management of the LNG IUS were invited by the Medical Department of the Brazilian affiliate of Bayer to take part in this Delphi panel on the practical recommendations on the use of the LNG IUS. We actively sought for technical referrals by asking gynecologists who they considered a reference in LARC and LNG IUS in their region. The participants were selected based on their expertise, large number of procedures performed, or academic background on the subject to compose a panel with physicians representing both public and private healthcare systems, from different Brazilian regions.
The panel was a Bayer initiative. An independent medical communication agency was hired to assist with the survey logistics and elaboration of questions. All the panelists had the opportunity to suggest questions and comments. The panelists answered two web-based anonymous questionnaires sent by the agency. The questionnaires were sent through a web-based platform (www. allcounted.com). The panelists did not receive any compensation to participate, and the sponsor did not have any access to the individual answers. The first round of questions was sent in January 2020 and the panelists had two weeks to answer the questions. The second round was sent in April 2020 and the panelists again had two weeks to answer it. No ethics committee approval is required for this kind of survey. The trade name Mirena® was adopted in the questions, as there is no generic or similar product in the Brazilian market. The authors of this report elaborated the questions, analyzed the answers, wrote, reviewed, and agreed with the final manuscript.
RESULTS
Panelists
Eighteen out of 20 gynecologists accepted to participate in the Panel. Most of them (14/18; 77.8%) had their medical degree for over 20 years. Only two were younger than 40 years old. The panelists were from the State of São Paulo (n=6), Paraná (n=3), Minas Gerais (n=2), Bahia (n=2), Goiás (n=2), Mato Grosso do Sul (nn=1), Brasília (n=1), and Espírito Santo (n=1). Twelve worked in both public and private healthcare systems; two worked only in the public health system and four worked only in the private setting. Among those who practiced in both settings, 10 dedicated more time to the private setting. All of them had complete residency in obstetrics and gynecology; 14 had a Master´s degree (eight of those also had a Ph.D. degree). All the 18 panelists answered to all the questions of the two rounds.
QUESTIONS AND RESPONSES
1) Which exams do you consider essential before inserting Mirena®?
The respondents could check more than one alternative. of the following alternatives: physical/gynecological exam; transvaginal ultrasound (TVUS), β-hCG, screening for sexually transmitted infections, Pap smear, vaginal and cervix culture, and Chlamydia/ gonorrhea culture. They could also answer “other alternative” and explain it.
In the first round of the panel 18 panelists (100%) considered physical/gynecological exam essential before inserting the device, seven (38.9%) considered transvaginal ultrasound (TVUS) and six (33.3%) voted for β-hCG and Pap smear; five panelists 27.8%) marked “other alternatives” (suggestions given by the panelists: to check menstrual profile, dyspareunia and the patient’s expectation; to rule out pregnancy but not necessarily with a β-hCG test, and to request β-hCG only in case the woman is not in the first 12 days of the menstrual cycle).
To rank which exams were considered the most important, we repeated the most voted alternatives in the second round, excluding physical/speculum examination. This time only three (16.7%) marked β-hCG and Pap smear as essential exams, while six (33.3%) marked TVUS as an essential exam (only one vote less in comparison with the first round). In the second round, we asked specifically if β-HCG was important only after the first 12 days of the cycle.
Therefore, in the opinion of the panelists, the essential exams before the insertion of Mirena® are physical and speculum examination followed by TVUS. However, there was no consensus regarding the need for the TVUS, as in the second round 6 out of 18 (33.3%) responded that it was essential.
2) Specific recommendations for nulligravidas
We made an open question “Do you have any specific recommendation for nulligravidas?” Eleven (61.1%) panelists had no specific recommendations. Seven (38.9%) reported the following recommendations: the need to use local anesthesia, complementary analgesia, wearing condoms in case of a new partner, screening for sexually transmitted infection (STI) in some cases, ruling out pregnancy, and checking for a minimum uterine length of 6 cm with TVUS to avoid the loss of the IUS in case the woman had a uterus size not compatible with Mirena®.
3) Minimum or maximum uterine length for the insertion of Mirena® and routine TVUS after the device insertion
Fifteen (83.3%) recommended minimum uterine length of 6cm for insertions and no maximum length. Some panelists noticed that it was possible to insert the LNG IUS in women with uterine length smaller than 6cm using TVUS to guide the procedure; one physician mentioned the higher risk of device expulsion in small uterus. Regarding the maximum uterine length to insert Mirena®, most panelists (13/18; 72.2%) did not consider any maximum value which could contraindicate the insertion of the device. Also, there were some recommendations for insertion in case of unusual uterine sizes, either larger or smaller. The respondents recommended that in cases of uterus size <6cm or >10cm always must correlate with the physical exam and in case of discordance perform TVUS to check for adequate positioning or the possibility of uterine perforation. In such cases, if possible, the TVUS should be performed immediately after the insertion or as early as possible, counsel woman about her risk of expulsion or the possibility of a different bleeding pattern. Overall, they recommend a more careful follow-up.
Regarding physicians who routinely ask for TVUS, eight (44.4%) panelists answered that the TVUS should be performed 30 days after the device placement. Five indicated that the exam must be performed immediately after LNG IUS placement or as soon as possible. Two recommended after 15 days and one panelist answered that she/he performs the exam when the woman returns to check the strings (3-12 weeks).
4) How long does the insertion procedure take?
We asked a question about the average time spent in the insertion of Mirena®, from the placement of the speculum until its removal. Twelve physicians (66.7%) answered 5 minutes and six (33.3%) answered 10 minutes. No one answered that the routine procedure could take longer than 10 minutes.
5) In addition to the general recommendations, what measures do you take to manage the pain and anxiety during and after the insertion procedure?
The majority prescribed nonsteroidal anti-inflammatory drugs (NSAIDs) before and after the insertion. In this question, the panelists could check more than one alternative. The responses were: analgesics, NSAIDs (12/18; 66.7%), anesthetic spray to the cervix, anesthetic cervical block, intrauterine anesthesia, sedation, non-pharmacological measures (e.g., relaxing music, forced cough) and others.
We made an open question about prescriptions after the insertion. Fifteen (83.3%) answered “yes”: 14 (77.8%) prescribed NSAIDs, one prescribed NSAID combined with scopolamine and one prescribed scopolamine.
6) Sexual abstinence and physical activities after insertion
The most frequent recommendation was to avoid sexual and physical activities in the first 24 hours after Mirena® insertion. Thirteen (72.2%) recommended sexual abstinence after the insertion of Mirena®. Seven recommended abstinences during the first 24 hours, three recommended it for 48 hours and three panelists recommended sexual abstinence for 3-7 days after the insertion. Regarding physical activities, 12 (66.6%) panelists answered that there is no need to wait to return to usual physical activities; however, five recommended waiting 24 hours before returning to physical activities and only one panelist recommended waiting 15 days before returning to physical activities.
7) How long should the woman wait to interrupt the previous contraceptive method?
Although the majority of panelists recommended stopping the previous contraceptive method immediately after the IUS insertion, there were conflicting answers. Eleven (61.1%) answered that the woman could interrupt the previous contraceptive method immediately after the insertion of Mirena®. However, four recommended the woman finish the blister of combined oral contraceptive (COC) and three recommended that it is necessary to wait 7-30 days before interrupting the previous method. In the second round, we asked specifically about COC and 10 physicians (55.5%) oriented women to interrupt COC immediately, five (27.8%) asked the woman to finish the blister and three (16.7%) recommended interrupting the COC seven days after the device placement.
Regarding current users of progestin-only pills, 10 (55.6%) recommended its immediate interruption, while six (33.3%) recommended finishing the blister and two (11.1%) recommended that the woman should maintain pill intake for at least seven days after the IUS placement. Regarding the use of a vaginal ring, the answers were conflicting; 10 respondents (55.6%) would recommend its interruption immediately and eight (44.4%) would ask the woman to maintain the use at least for seven days after device insertion.
8) How long after the insertion should the first follow-up visit take place?
Twelve (66.7%) panelists reported that the first follow-up visit could occur between 20 and 60 days after the device insertion. Three suggested “after the TVUS”, and three suggested a period between three and six months.
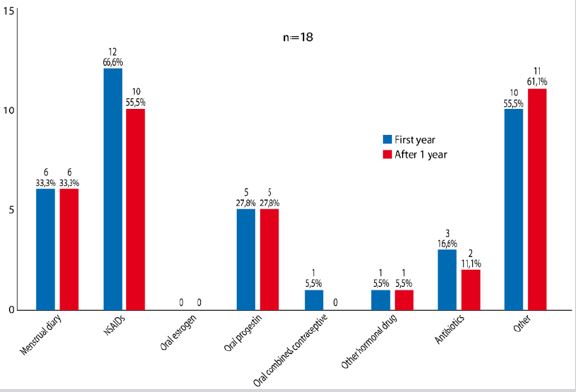
9) Management of abnormal uterine bleeding immediately after device placement and outside the adaptation period (i.e, one year after the insertion).
The panelists were asked about the management of abnormal uterine bleeding (AUB) that could occur just after the insertion. Multiple choices were allowed (Figure 1). NSAIDs were the most commonly prescribed drugs to manage the AUB; they were prescribed by 12 (66.7%) panelists, followed by oral progestin (n=5; 27.8%). In the “other” alternative, 6/10 panelists indicated they prescribed tranexamic acid, 3/10 reported they had an expectant attitude and counseling and one prescribed Omega-3. The menstrual diary calendar was adopted as a follow-up tool by six physicians (33.3%).
In the management of AUB outside the adaptation period (i.e, one year after the insertion), again the most prescribed drugs were NSAIDs (n=10/18; 55.5%) (Figure 1). As many panelists (38.9%) answered that they prescribe hormonal drugs to manage AUB, either in the adaptation period or after it, we made an open question about this therapeutic conduct in the second round. The most cited reason was that continuous oral progestin or natural progesterone were useful to stop AUB n= 7; 38.9%). Regarding exams required for women with AUB, they recommended TVUS, in addition to a careful gynecological exam. Indeed, they also reported that TVUS was not mandatory and should be decided on an individual basis.
10) Routine recommendation for follow-up after Mirena® insertion.
Eleven respondents (61.1%) recommended routine TVUS (9 recommended it yearly and 2 recommended every 6 months) and two panelists recommended that the woman should also be oriented to check the strings by herself. Overall, they reported that women might return once a year for follow- up.
11) How to manage a pregnancy occurring with Mirena® in situ?
Although it is an uncommon situation, some panelists answered based on their experience with other intrauterine devices. The majority (16; 88.9%) answered that they would try to remove the device; however, 11 would try to remove it only in specific cases. Half of the panelists would not change the antenatal routine care.
12) If an ectopic pregnancy has occurred while in use of Mirena®, what measures do you take regarding the use of Mirena® after this event?
If Mirena® continues to be the contraceptive method chosen by the woman after an ectopic pregnancy with Mirena® in situ, 10 (55.6%) reported that they would maintain the same device and two (11.11%) would replace the current Mirena® by a new one. Three (16.7%) would no longer use Mirena® and 3 (16.7%) would re-evaluate the case.
13) When do you consider Mirena® malpositioned and how do you manage it and a device expulsion?
All panelists agree that Mirena® is malpositioned when it is below the internal cervical os. Five (27.8%) respondents also consider that Mirena® is in malposition when it is below the external cervical os and 2 (11.1%) when it is in an unusual position in the uterine cavity (e.g., in a transversal position). Furthermore, 10 (55.5%) of the respondents consider that it is mandatory to remove Mirena® and substitute it for a new one, whereas one panelist inserts the same IUS. Also, in the case of device expulsion, all the panelists would try a new insertion. Eight would consider not trying again after two attempts, and three would give up after three attempts.
14) What to do when a user of Mirena® wants to become pregnant.
Ten (55.5%) answered that the woman can start to try to get pregnant as soon as Mirena® is removed. Eight recommend the woman take folic acid for three months before removing Mirena®.
15) What is the age range for the insertion of Mirena®? What do you do after menopause?
The majority (17; 94.4%) considered that there was no minimal neither maximal age after menarche required to use Mirena®. Regarding what to do with women who reached menopause with Mirena® in situ, all the panelists reported that they keep it. Fifteen (83.3%) mentioned that Mirena® could be useful as an add-on to estrogen hormone therapy. Also, all the panelists agree that there is no need to remove Mirena®, as it can protect the endometrium and is a good contraceptive in the transition period to menopause.
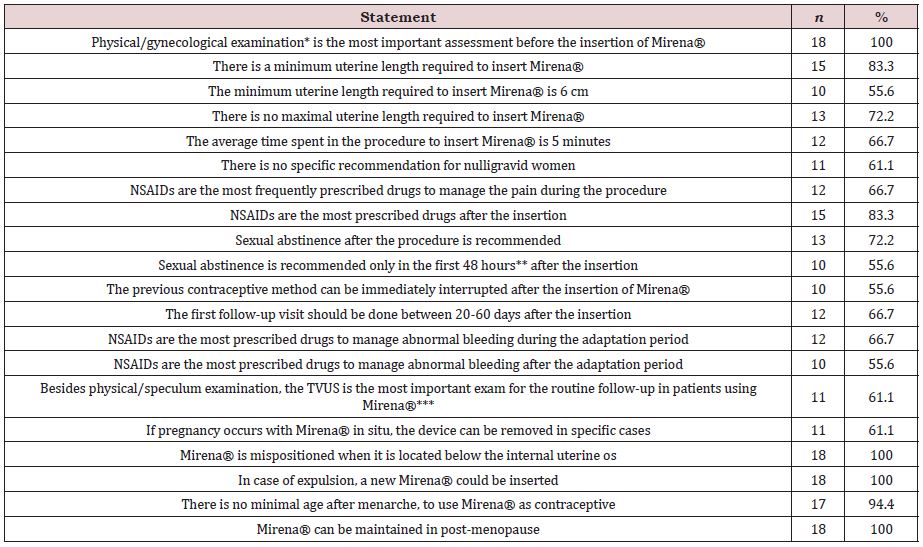
The Table 1 summarizes the main findings of the Panel.
DISCUSSION
The experience shared by the panelists reflects their routine practice and by correlating them with the current guidelines, we may help clinicians to make an informed decision regarding common aspects related to the LNG IUS use. This information could also be important for the management of other IUS, such as the new low-dose LNG IUS (Kyleena®) recently introduced in the Brazilian market. Although this new device is smaller than Mirena®, the insertion, management and safety profile are quite similar [13].
Although the panelists were physicians with large experience in the insertion and management of the LNG IUS, some answers were unexpected, with divergent conduct.
Lack of training by the physicians, lack of knowledge by women, costs and access to healthcare are the main barriers to wider use of LNG -IUS [8,9]. The exams requested by one-third of the panelists (β-hCG, TVUS, and Pap smear) before the device insertion might increase costs unnecessarily or even demotivate the woman in many cases. The Pap smear could be done in the first visit after the insertion, and β-hCG is only recommended in case of uncertainty if the woman is pregnant or not. In general, TVUS is not helpful before the IUS placement [14].
When the panelists considered the management of the insertion in nulligravida, despite having no specific orientations, more than one third recommended the intracervical block or complementary analgesia. Although some physicians perceive the insertion as more painful and difficult in nulligravidas, the literature shows that the procedure is simple and well-tolerated by this group [15,16]. In general, there is no clear guidance and besides the use of lidocaine as intracervical block for nulligravidas, no treatment for pain has shown superiority over others [17]. Most participants reported the use of NSAIDs before and after the insertion. Evidence shows that the fear of the insertion procedure can prevent many women from choosing this method [15], therefore, we consider valid any strategy to reduce pain at the insertion, as long as they do not become a barrier to the use of LNG IUS, given the fact that most women do not require anesthesia.
Some participants wait before abandoning previous contraceptive methods. It is well established that the onset of action of the LNG IUS is immediately after placement, nonetheless the possibility of ovulation and conception before using this product must be considered, especially if the insertion is performed during the luteal phase and the provider have doubts if the woman is pregnant.
According to the product label, in women of fertile age, Mirena® should be inserted into the uterine cavity within seven days of the onset of menstruation. In this case, no backup contraception is needed. Mirena® can be inserted any time during the menstrual cycle if the physician can be reasonably certain (as defined by the World Health Organization) [14] that the woman is not pregnant. After the seven days and when no other contraceptive method is used or switching from copper IUD, international guidelines recommend the use of a barrier contraceptive method or abstain from vaginal intercourse for the next seven days. When switching from pills, patch and vaginal ring, they must be continued for seven days after the insertion procedure. When switching from other hormonal IUD or subdermal implant, the insertion and removal procedure must be done at the same time, this way there is no need for backup contraception. If the previous method was an injectable, the insertion must occur when the repeat injection would have been given. Since the product label does not address every switching possibility and locally there is a lack of clear guidance on the matter, we could observe a vast array of strategies [14,18,19].
The routine use of TVUS in the follow-up was a constant in the answers of the panelists, and some reported that TVUS should be performed 30 days after the IUS placement while others recommended the exam to be performed immediately after the procedure. There is no evidence that TVUS is necessary unless the physician has doubts about the device positioning or abnormal pain described by the user. Nonetheless, this practice is widely used and might be adequate when there is easy access. It is important to emphasize that the difficult access to perform a TVUS should not be a barrier to the insertion of the LNG IUS.
Two-thirds recommended the first follow-up within the first 60 days after insertion, which is an excellent recommendation because most expulsions (50%) occur within the first 6 months after device placement [20,21]. However, it is important to take into account that besides this initial period, the follow-up for LNG-IUS must be done annually. The evidence shows that additional visits do not reduce the possibility of expulsion [22]. Frequent appointments only to check an IUD could give the impression that its use requires frequent medical attention and ultimately end up creating anxiety among users.
One question that generated conflicting answers was how to deal with women with AUB after Mirena® placement. One of the side-effects induced by the use of Mirena® is AUB, which commonly occurs within the first six months after placement; however, it could occur at any time and could be a cause of premature discontinuation although this side-effect was described in a proportion lower than expected [23]. There is enough information that there is no effective treatment, although tranexamic and mefenamic acid were tested [24,25]. In addition, recently ulipristal acetate (UPA) at the dose of 5mg/5 days was compared to placebo, showing a trend of superiority over placebo in the bleeding control [26]; however, the drug is not available in Brazil. Although one-third of the panelists reported the use of COC or progestin-only pills to bleeding control, it is not supported by the evidence because it is well described that the endometrium in users of Mirena® is refractory to estrogens [27,28].
The strength of our study was the participation of physicians with large experience with Mirena® providing honest, independent and anonymous opinions, working in both private and public healthcare settings and located in different regions of Brazil. The limitations were that the panelists were not randomly selected, and the number of respondents was possibly low, although there are no clear guidelines suggesting the numbers to be included in studies applying the Delphi survey (suggested sample sizes range from 10 to 50) [29]. The questionnaire could not be extremely long because the attention reduces over time and we cannot exclude the courtesy bias induced by the invitation itself, although it was minimized by the fact that no questions compared Mirena® with other LARCs in terms of efficacy or safety.
In conclusion, most aspects related to Mirena® usage reached an agreement in terms of clinical practice among physicians of different backgrounds and also tend to correlate to the available guidelines. However, despite the availability of LNG IUS in Brazil for many years and the large experience of the panelists, there is still some disagreement regarding routine practices (e.g., pre-insertion exams). There is a need for continuous education of physicians and the standardization of certain procedures to expand the use of this contraceptive method.
CONTRIBUTIONS
LB, ALSF and TEU designed the study, elaborated the questions, analyzed the results, wrote and reviewed the manuscript.
ACKNOWLEDGMENT
We thank the 18 panelists who kindly responded to the survey. The panelists did not receive any payment.
This study was supported by Bayer Brazil, through the payment of an independent medical communication agency (Triple Check Consulting).
CONFLICTS OF INTEREST
LB and ALSF did not receive any compensation for this particular manuscript, but they have received honorarium from Bayer Brazil in the last 12 months, outside the scope of this article. TEU is an employee of Bayer.
REFERENCES
- Espey E, Ogburn T (2011) Long-acting reversible contraceptives: Intrauterine devices and the contraceptive implant. Obstet Gynecol 117(3): 705-719.
- Machado RB, Maria I, Monteiro U, Magalhães J, Aparecida C, et al. (2017) Long-acting reversible contraception. Contracepção reversível de longa ação. Rev Bras Ginecol Obs 39(6): 294-308.
- Finotti MF, Magalhães J, Martins L, Franceschini SA (2018) Métodos anticoncepcionais reversíveis de longa duração. Comissão Nacional Especializada em Anticoncepção, Protocolo FEBRASGO no. 71, São Paulo, Brazil.
- Lähteenmäki P, Rauramo I, Backman T (2000) The levonorgestrel intrauterine system in contraception. Steroids 65 (10-11): 693-697.
- Bahamondes L, Fernandes A, Monteiro I, Bahamondes MV (2020) Longacting reversible contraceptive (LARCs) methods. Best Pract Res Clin Obstet Gynaecol 66: 28-40.
- Heinemann K, Reed S, Moehner S, Do Minh T (2015) Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: The European active surveillance study for intrauterine devices. Contraception 91(4): 280-283.
- Bahamondes L, Fernandes A, Monteiro I (2017) Barriers to Implementing and Consolidating a Family Planning Program that would meet Brazilian Needs. Rev Bras Ginecol e Obstet 39(8): 373-375.
- Rademacher KH, Sripipatana T, Pfitzer A, Mackay A, Thurston S, et al. (2018) A global learning agenda for the levonorgestrel intrauterine system (LNG IUS): Addressing challenges and opportunities to increase access. Glob Heal Sci Pract 6(4): 635-643.
- Sweeney LA, Molloy GJ, Byrne M, Murphy AW, Morgan K, et al. (2015) A qualitative study of prescription contraception use: The perspectives of users, general practitioners, and pharmacists. PLoS One 10(12): e0144074.
- Middleton AJ, Naish J, Singer N (2011) General practitioners’ views on the use of the levonorgestrel-releasing intrauterine system in young, nulligravid women, in London, UK. Eur J Contracept Reprod Heal Care 16(4): 311-318.
- Marques JBV, Freitas D de, Marques JBV, Freitas D de (2018) Método DELPHI: Caracterização e potencialidades na pesquisa em Educação. Pro-Posições 29: 389-415.
- Hohmann E, Brand JC, Rossi MJ, Lubowitz JH (2018) Expert opinion is necessary: Delphi panel methodology facilitates a scientific approach to consensus. Arthrosc J Arthrosc Relat Surg 34(2): 349-551.
- McKeage K, Lyseng-Williamson KA (2017) KyleenaTM (levonorgestrelreleasing intrauterine system) in contraception: a profile of its use. Drugs Ther Perspect 33(5): 202-207.
- World Health Organization (WHO) (2016) Selected practice recommendations for contraceptive use.
- De Nadai MN, Poli-Neto OB, Franceschini SA, Yamaguti EMM, Monteiro IMU, et al. (2020) Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: A randomized double-blind controlled trial. Am J Obstet Gynecol 222(3): 245.
- Bahamondes MV, Hidalgo MM, Bahamondes L, Monteiro I (2011) Ease of insertion and clinical performance of the levonorgestrel-releasing intrauterine system in nulligravidas. Contraception 84(5): e11-6.
- Gemzell-Danielsson K, Jensen JT, Monteiro I, Peers T, Rodriguez M, et al. (2019) Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: An updated review. Acta Obstet Gynecol Scand 98(12): 1500-1513.
- Reproductive Access Health Project (2015) How to switch birth control methods.
- American Family Physician (2011) Information from your family doctor how to switch birth control methods. Am Fam Physician 83: 575.
- Curtis KM, Jatlaoui TC, Tepper NK, Zapata LB, Horton LG, et al. (2016) US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Reports 65(4): 1-66.
- National Institute for Health and Care Excellence (NICE) (2019) Longacting reversible contraception. NICE Guidance.
- Bahamondes L, Monteiro I, Fernandes A, Gaffield ME (2019) Follow-up visits to check strings after intrauterine contraceptive placement cannot predict or prevent future expulsion. Eur J Contracept Reprod Heal Care 24(2): 97-101.
- Heikinheimo O, Inki P, Scheltmer T, Gemzell-Danielsson K (2014) Bleeding pattern and user satisfaction in second consecutive levonorgestrelreleasing intrauterine system users: Results of a prospective 5-year study. Hum Reprod 29(6): 1182-1188.
- Sørdal T, Inki P, Draeby J, O’Flynn M, Schmelter T (2013) Management of initial bleeding or spotting after levonorgestrel-releasing intrauterine system placement: A randomized controlled trial. Obstet Gynecol 121(5): 934-941.
- Gemzell-Danielsson K, Inki P, Boubli L, O’Flynn M, Kunz M, et al. (2010) Bleeding pattern and safety of consecutive use of the Levonorgestrel- Releasing Intrauterine System (LNG-IUS)- A multicenter prospective study. Hum Reprod 25(2): 354-359.
- Fava M, Peloggia A, Baccaro LF, Castro S, Carvalho N, et al. (2020) A randomized controlled pilot study of ulipristal acetate for abnormal bleeding among women using the 52‐mg levonorgestrel intrauterine system. Int J Gynecol Obstet 149(1): 10-15.
- Dinehart E, Lathi RB, Aghajanova L (2020) Levonorgestrel IUD: Is there a long-lasting effect on return to fertility? J Assist Reprod Genet 37(1): 45-52.
- Pengdi Z, Hongzhi L, Ruhua X, Jie C, Shangchun W, Juhua C, et al. (1989) The effect of intrauterine devices, the stainless-steel ring, the copper T220, and releasing levonorgestrel, on the bleeding profile and the morphological strucuture of the human endometrium - A comparative study of three IUDs. Contraception 40(4): 425-438.
- Shariff NJ (2015) Utilizing the Delphi survey approach: A review. J Nurs Care 4: 1-6.
Article Type
Case Report
Publication history
Received Date: October 15, 2021
Published: November 12, 2021
Address for correspondence
Thaís Emy Ushikusa, Medical Affairs of Women’s Health Care Department, Bayer SA, Brazil
Copyright
©2021 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Luis B, Agnaldo Lopes da Silva F, Thais Emy U. Practical Recommendations on the Management of Issues Related to the Levonorgestrel 52mg Intrauterine System: A Delphi Panel. 2021- 3(6) OAJBS.ID.000347.