Pharmacokinetics in Critically Ill Patients, What Changes?
ABSTRACT
Background: Pharmacokinetics is defined as a mechanism or set of mechanisms by which the body interacts with the substances
administered during the entire duration of drug exposure. The components of pharmacokinetics are absorption, distribution,
metabolism, and excretion. All patients suffering from life-threatening illnesses must receive care in an intensive care unit. In this
unit, it is not only necessary to have equipment that can provide comprehensive care to the patient, but also to have staff trained to
act in any clinical environment.
Methodology: A systematic review was carried out through various databases from January 2014 to February 2022; The search
and selection of articles was carried out in indexed journals in English.
Results: The understanding of the pharmacokinetics in the critical patient is of vital importance, being the pillar of this
understanding the patient and providing adequate care and management. The cornerstones of intensive care management are the
optimization of the patient’s physiology, the provision of advanced organ support, and the identification and treatment of underlying
disease processes. In antibiotic therapy it is necessary to optimize the dosage and improve the route of administration. Table 1
reports the main pharmacokinetic changes.
Conclusion: This review offers up-to-date and detailed information on the pharmacokinetics in critically ill patients with the
aim of providing “personalized” care depending on the patient’s physiological state.
KEYWORDS
Pharmacokinetics; Pharmacodynamics; Critical patient; sepsis; antibiotic therapy
INTRODUCTION
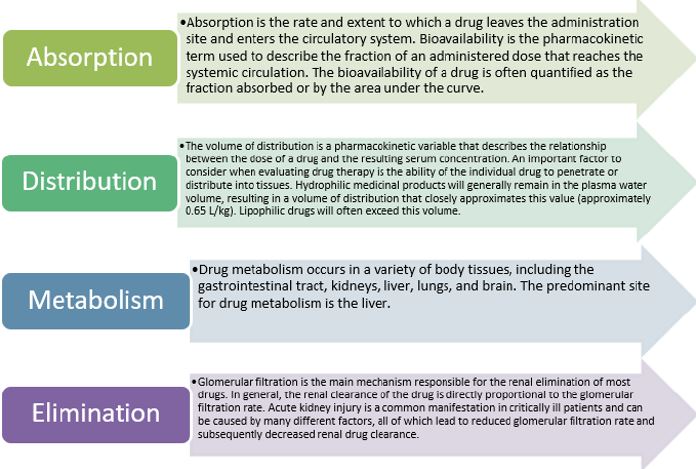
Pharmacokinetics is defined as a mechanism or set of mechanisms by which the body interacts with the substances administered during the entire duration of drug exposure. This term tends to be used or associated with pharmacodynamics, but its definition is totally different, since it deals with the effect of the drug in the body [1]. In order to have a better understanding of pharmacokinetics, it is necessary to analyze and study all its components, as expressed below in an orderly manner: first, absorption; second, distribution; third, metabolism; and fourth, excretion [2]. These four components are necessary when administering a drug to the patient, in order to provide the lowest possible risk, optimizing its therapeutic part, making the necessary adjustments given the varied physiology and lifestyles of each patient.
Absorption is defined as the process of taking a drug from administration, e.g., tablet, capsule, to the systemic circulation. This can modify or affect the speed and concentration at which a drug can reach its desired site of effect. Therefore, it is important to analyze the bioavailability of each patient, being defined as the fraction of the originally administered drug that reaches the systemic circulation and depends on the properties of the substance and the mode of administration [2]. It may be a direct reflection of drug absorption. For example, when a drug is administered intravenously, 100% of the drug reaches the circulation virtually instantaneously, giving this method 100% bioavailability [3].
Distribution describes how a substance spreads throughout the body. This varies depending on the biochemical properties of the drug, as well as the physiology of the individual taking that drug. This is influenced by the diffusion and convection of the drug. The volume of distribution is a common method to describe the diffusion of a drug. It is defined as the amount of drug in the body divided by the plasma concentration of the drug [4]. The distribution has an important section, which is the protein binding, since the drug can be bound to proteins or free. Only the free drug can act at its pharmacologically active sites, e.g., receptors, cross into other fluid compartments, or be eliminated [4,5]. In the clinical setting, the free concentration of a drug at plasma receptor sites correlates more closely with effect than the total plasma concentration. Metabolism is the body’s processing of the drug into subsequent compounds. This is often used to convert the drug to more water soluble substances that will progress to renal clearance or, in the case of prodrug administration such as codeine, metabolism may be required to convert the drug to active metabolites [6]. Excretion is the process by which the drug is removed from the body. Being the kidney the main organ that is in charge, but it can also be eliminated through the lungs, through the skin or the gastrointestinal tract.
All patients who suffer from life-threatening diseases or may be at risk of developing them must receive care in an intensive care unit, since it is the unit capable of providing the correct care [7]. In this unit, high proportions of personnel can be offered, in order to care for the critically ill patient, providing advanced monitoring and organ support in order to improve the morbidity and mortality of patients [8]. In this unit it is not only necessary to have teams that can provide comprehensive care to the patient, but also to have staff trained to act in any clinical environment, the first steps being to contribute to the prevention of the disease, to be alert to a response providing a multidisciplinary approach before, during the stay in the ICU, and after the ICU, providing comprehensive followup or good quality palliative care [9]. The cornerstones of intensive care management are the optimization of the patient’s physiology, the provision of advanced organ support, and the identification and treatment of underlying disease processes [10]. Therefore, it is necessary and pertinent to carry out this work, in order to provide updated and accurate information on pharmacokinetics in critically ill patients, providing “personalized” care depending on the physiological state of the patient.
MATERIALS AND METHODS
A systematic review was carried out. The databases used are PubMed, Scielo and ScienceDirect, Google scholar, among others. Indexed articles in English-language journals from the years 2014 to 2022 were chosen. The keywords were chosen taking into account the DeCS and MeSH methodology, the terms are: Pharmacokinetics; Pharmacodynamics; Critical patient; sepsis; antibiotic therapy. In this review, 102 original and review publications related to the subject studied were identified, of which 25 articles met the specified inclusion requirements, such as articles that were in a range of not less than the year 2014, that were articles from full text and inform about the subject. As exclusion criteria, it was taken into account that the articles did not have sufficient information and that they did not present the full text at the time of their review.
RESULTS
Pharmacokinetics
The understanding of pharmacokinetics in the critical patient is of vital importance, being the pillar of this understanding the patient and providing adequate care and management [11]. For a correct implementation of this, it is necessary to individualize management in critically ill patients, since the implementation of a medication that is not in accordance with the patient’s conditions can cause serious adverse events and such events lead to a longer length of stay and higher costs [12].
ICU physicians require a detailed understanding of drug pharmacokinetics and pharmacodynamics to ensure safe and effective drug regimens. The lack of anticipation and monitoring of changes in the pharmacokinetics and pharmacodynamics of a drug in critically ill patients can contribute to clinical failures or adverse reactions [13]. As is known, pharmacokinetics describes the movement of a drug through the body and is divided into four main components as we can see in Figure 1; [13-15].
With respect to absorption, numerous drug-specific variables can influence its absorption, including particle size, solubility, lipophilicity, ionization, and dissociation rate constant of the drug. Factors that influence the gastrointestinal tract can also alter drug absorption, such as gastric pH, regional blood flow, surface area, and motility [16].
The Critically Patient and Pharmacokinetic Changes
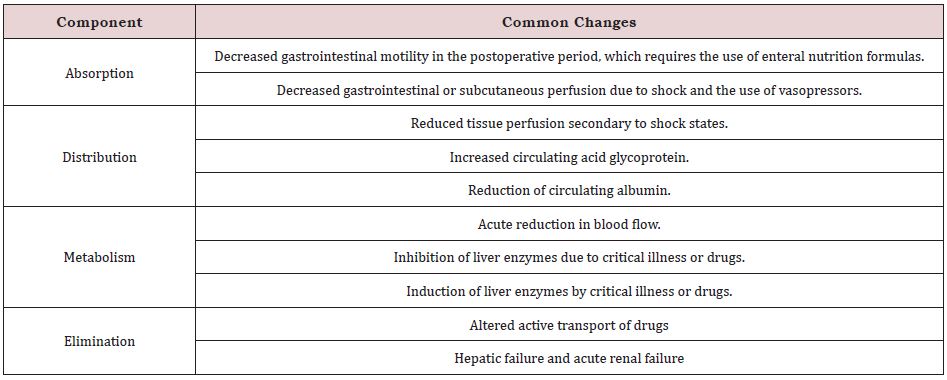
Despite the regular use of medications for critically ill patients, overall data is limited regarding the impact of critical illness on pharmacokinetics. Thus, the design of safe and effective drug regimens for critically ill patients requires an understanding of pharmacokinetics. To date, some pharmacokinetic changes are known, which we will present in Table 1, which will help us to have a better understanding [14-17].
Absorption
a) Use of vasopressors: Studies have shown that vasopressors, including dopamine, epinephrine, and norepinephrine, have different effects on both splanchnic perfusion and gastrointestinal oxygen consumption [17]. It has been verified that the absorption of paracetamol in critically ill patients who received dopamine had a lower area under the curve than in patients who did not receive dopamine. Although these data are not entirely clear, due to the general lack of data evaluating the impact of vasoactive drugs on gastrointestinal drug absorption. One of the measures to be able to implement the correct dose with the desired effect in the critical patient is to use the intravenous route, thus the health professional will be more sure of the desired therapeutic effect. Limited data with low molecular weight heparins suggest reduced subcutaneous absorption associated with vasopressor administration, and it is important that healthcare professionals are aware of this potential.
b) Delayed gastric emptying: Reasons for delayed or slow gastric emptying include surgery, postoperative ileus, trauma, head injury, burns, sepsis, and opioid use. Because most drugs are absorbed through the small intestine, delayed gastric emptying will delay the time to peak concentration and delay the onset of a drug’s action [18].
c) Interactions between feeding tube and nutrients: Enteral feeding solutions can increase the pH of the stomach, which reduces the absorption of medications that require an acidic environment. Several ingredients in the enteral feeding solution may also bind directly to some medications and reduce absorption. Phenytoin and ciprofloxacin are examples of drugs that exhibit variable decreased absorption in the presence of enteral feeding solutions [19]. These drug interactions can be managed with enteral feeding solutions by withholding the feeding solution 1 to 2 hours before and after drug administration, or the drug can also be administered intravenously.
Distribution
a) Fluid resuscitation: It is an essential intervention used in many critically ill patients. The infused fluid leads to an increase in total body water, resulting in a decrease in the serum concentration of hydrophilic drugs. Due to vasodilator shock, these effects are aggravated by decreasing the drug in plasma. Failure to achieve effective serum drug concentrations can potentially lead to treatment failure and possibly induce antimicrobial resistance.
b) Plasma protein binding: The predominant plasma proteins to which the drugs bind are albumin and α-1 acid glycoprotein. Acidic drugs, such as phenytoin, tend to bind to albumin, while basic drugs, such as lidocaine, tend to bind to acid glycoprotein. In critically ill patients, many conditions can lead to changes in plasma protein levels. Increased vascular permeability and protein catabolism may result in decreased albumin concentrations due to acute stress or trauma.
c) Tissue perfusion: Regional tissue hypoperfusion is a common manifestation in many critically ill patients. Systemic hemodynamic changes can have an adverse impact on tissue perfusion, resulting in decreased delivery of hydrophilic drugs by blood to the capillary. This impaired delivery to tissues can limit the efficacy of the drug at the site of action, especially for non-central organs and peripheral tissues [19,20].
Metabolism
a) Liver enzyme activity: Disease states found in the ICU may affect the activity of metabolic enzymes [21]. Alterations in CYP450 activity may result in prolonged or reduced therapeutic effects of parent compounds, an increased load of toxic metabolites, or a delayed response of prodrugs that require hepatic enzymes to become pharmacologically active. In severe burns, the activity of the CYP450 enzyme system can be significantly decreased [22].
b) Hepatic blood flow: Disease states and therapies can alter hepatic blood flow, impairing the hepatic clearance of drugs with high extraction ratios. During severe sepsis and septic shock, for example, cardiac output may increase (early stage) or decrease (late stage), leading to disturbances in hepatic blood flow [23]. In the setting of cirrhosis, hepatic blood flow can be substantially decreased as a result of intrahepatic and extrahepatic portalsystemic shunting. The use of medications in the ICU may also have an impact on hepatic blood flow. Vasopressors, such as norepinephrine, epinephrine, and phenylephrine, have been shown to reduce hepatic blood flow, which is thought to be induced by adrenergic-mediated vasoconstriction of the hepatic artery and portal vein. Conversely, vasodilators, such as nitroglycerin and nitroprusside, can increase hepatic blood flow by reducing hepatic vascular resistance [24,25].
DISCUSSION
The understanding of the pharmacokinetics in the critical patient is of vital importance, being the pillar of this understanding the patient and providing adequate care and management. The cornerstones of intensive care management are the optimization of the patient’s physiology, the provision of advanced organ support, and the identification and treatment of underlying disease processes. The study by Mohd et al, informs us of one of the therapeutic approaches that must be taken into account in critically ill patients, which is antibiotic therapy, although over the years bacterial resistance has been increasing in antibiotic therapy approaches. They have also been modified depending on the type of resistance. Therefore, even more care and special attention should be given to critical patients. There is little evidence that demonstrates the efficacy of antibiotic therapy in critically ill patients, so experts recommend administering the highest tolerated antibiotic therapy dose through alternative dosing strategies, considering the combined use of multiple antibiotics, which are rationally optimized, particularly when early course of infection in critically ill patients. (24).
This study is supported by the work carried out by Snehal et al, when reporting the multi-organ changes that can occur in critically ill patients, such as capillary leak, increased cardiac output and altered protein levels that can have profound effects on the volume of distribution and clearance of antibacterial agents directly or indirectly affecting pharmacokinetics and pharmacodynamics. Therefore, they recommend optimizing and seeking new dosing and administration strategies in order to adapt to the pharmacokinetics of the critical patient individually and thus increase antibacterial exposure. (25).
These studies confirm the changes that occur in the critically ill patient, so their dosing schemes should be optimized individually, although studies are still needed to verify the optimization of antibiotic therapy in the critically ill patient. A strength of the current study is the methodology implemented, regarding the literature search, and steps in the selection of relevant articles, quality assessment, and data extraction. However, this study has several limitations, which should be taken into account before reaching a conclusion, among these are the little evidence from the analysis of clinical trials that demonstrate the efficacy of pharmacotherapy when optimizing doses in critically ill patients, Therefore, more studies are needed to answer these questions.
CONCLUSION
The understanding of the pharmacokinetics in the critical patient is of vital importance, being the pillar of this understanding the patient and providing adequate care and management. Despite the regular use of medications for critically ill patients, overall data is limited regarding the impact of critical illness on pharmacokinetics. Table 1 shows the main pharmacokinetic changes that can occur in critically ill patients. The cornerstones of intensive care management are the optimization of the patient’s physiology, the provision of advanced organ support, and the identification and treatment of underlying disease processes. With regard to antibiotic therapy in the critically ill patient, it is necessary to optimize the dosage and improve the route of administration of the critically ill patient in order to have greater antibacterial exposure.
REFERENCES
- Zhivkova ZD, Mandova T, Doytchinova I (2015) Quantitative Structure- Pharmacokinetics Relationships Analysis of Basic Drugs: Volume of Distribution. J Pharm Pharm Sci 18(3): 515-527.
- Mei H, Wang J, Che H, Wang R, Cai Y (2019) The clinical efficacy and safety of vancomycin loading dose: A systematic review and metaanalysis. Medicine (Baltimore) 98(43): e17639.
- Gray K, Adhikary SD, Janicki P (2018) Pharmacogenomics of analgesics in anesthesia practice: A current update of literature. J Anaesthesiol Clin Pharmacol 34(2): 155-160.
- Westervelt P, Cho K, Bright DR, Kisor DF (2014) Drug-gene interactions: inherent variability in drug maintenance dose requirements. PT 39(9): 630-637.
- Lin HS, Watts JN, Peel NM (2016) Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 16(1): 157.
- Dmitri N, Aneel B, James CG, Elizabeth L, Omar MO (2020) Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 396: 27-38.
- (2020) ICNARC report on COVID-19 in critical care.
- Malbrain ML, Marik PE, Witters I (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46(5): 361-380.
- Gonzalo H, Oriol R, Colinas L (2017) High-flow nasal cannula support therapy: new insights and improving performance. Crit Care 21(1): 62.
- Singer M, Deutschman CS, Seymour CW (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). J Am Med Assoc 315(8): 801-810.
- Heffernan A, Sazlly S, Lipman J, Roberts J (2021) A personalized approach to antibiotic pharmacokinetics and pharmacodynamics in critically ill patients. Anaesth Crit Care Pain Med 40(6): 100970.
- D’Avolio A, Pensi D, Baietto L (2016) Daptomycin pharmacokinetics and pharmacodynamics in septic and critically ill patients. Drugs 76(12): 1161-1174.
- Hao JJ, Chen H, Zhou JX (2016) Continuous versus intermittent infusion of vancomycin in adult patients: A systematic review and meta-analysis. Int J Antimicrob Agents 47(1): 28-35.
- Jeffres MN (2017) The whole price of vancomycin: Toxicties, troughs and time. Drugs 77(11): 1143-1154.
- Kovacˇevic´ T, Avram S, Milakovic´ D (2016) Therapeutic monitoring of amikacin and gentamicin in critically and noncritically ill patients. J Basic Clin Pharm 7(3): 65-69.
- Allou N, Allyn J, Levy Y (2016) Assessment of the National French recommendations regarding the dosing regimen of 8mg/kg of gentamicin in patients hospitalized in intensive care units. Anaesth Crit Care Pain Med 35(5): 331-335.
- Roberts JA, Abdul-Aziz MH, Lipman J (2014) Individualized antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect Dis 14: 498-509.
- Wong WT, Choi G, Gomersall CD, Lipman J (2015) To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: The eternal doubt. Curr Opin Pharmacol 24: 68-78.
- Hahn J, Choi JH, Chang MJ (2017) Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther 42(6): 661-671.
- Denny KJ, Cotta MO, Parker SL (2016) The use and risks of antibiotics in critically ill patients. Expert Opin Drug Saf 15(6): 667-678.
- Roger C, Nucci B, Molinari N (2015) Standard dosing of amikacin and gentamicin in critically ill patients results in variable and subtherapeutic concentrations. Int J Antimicrob Agents 46(1): 21-27.
- Droege ME, Van Fleet SL, Mueller EW (2016) Application of antibiotic pharmacodynamics and dosing principles in patients with sepsis. Crit Care Nurse 36(2): 22-32.
- Minichmayr IK, Schaeftlein A, Kuti JL (2017) Clinical determinants of target non-attainment of linezolid in plasma and interstitial space fluid: A pooled population pharmacokinetic analysis with focus on critically ill patients. Clin Pharmacokinet 56(6): 617-633.
- Abdul M, Lipman J, Mouton J, Hope W, Roberts J (2015) Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin Respir Crit Care Med 36(1): 136-53.
- Shah S, Barton G, Fischer A (2015) Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc 16(2): 147-153.
Article Type
Research Article
Publication history
Received Date: December 06, 2022
Published: February 22, 2023
Address for correspondence
Cindy Paola Cerro Martinez, Gynecologist, Universidad Libre, Colombia; https://orcid.org/0000-0001-9915-1789
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Cindy Paola CM, Said Samir SS, Francisco Alberto RL, Jorge Andrés MS, Carlos Jose CH, et. al. Pharmacokinetics in Critically Ill Patients, What Changes?. 2023- 5(1) OAJBS. ID.000549.