Myelosuppression and Other Adverse Reactions Caused by Linezolid
ABSTRACT
Background: Linezolid is a synthetic oxazolidinone antimicrobial drug. It is indicated for gram-positive infections and approved
for the treatment of other conditions. Myelosuppression is a condition in which bone marrow activity decreases, resulting in fewer
red blood cells, white blood cells, and platelets. Pancytopenia is not a disease but a manifestation of other underlying conditions.
Methodology: A systematic review was carried out through various databases, such as PubMed, Scielo and ScienceDirect; The
search and selection of articles was carried out in indexed journals in English.
Results: Linezolid is used as an effective antibiotic against bacteria belonging to the gram-positive group. At high doses up to
1,000 mg/kg/day, reversible myelosuppression occurred. This antibiotic also presents other adverse reactions to take into account
such as serotonin syndrome, hypoglycemia, among others.
Conclusion: This review offers up-to-date and detailed information on the adverse effects and hematological effects of Linezolid
in order to identify its correct use as well as its prognosis.
KEYWORDS
Myelosuppression; Adverse effects; Linezolid; Pediatric
INTRODUCTION
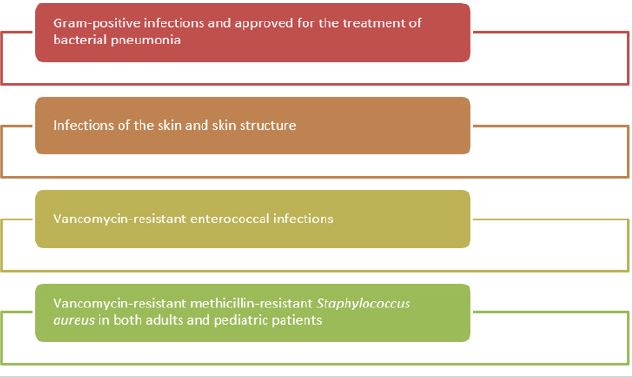
Linezolid is a synthetic oxazolidinone antimicrobial drug. It is indicated for gram-positive infections and approved for the treatment of bacterial pneumonia, skin and skin structure infections, and vancomycin-resistant enterococcal infections, including infections complicated by bacteremia [1,2]. Linezolid is not yet approved for the treatment of catheter-site infections, catheter-related bloodstream infections, and gram-negative infections. Linezolid’s primary locus of therapy is as an alternative to vancomycin in inpatient settings [3]. Vancomycin remains a standard treatment for methicillin-resistant Staphylococcus aureus infection. It has been seen and documented that there are bacteria such as Staphylococcus aureus that are resistant to vancomycin, so the use of Linezolid would be indicated, but it must be taken into account that inappropriate use of Linezolid can lead to resistance to this drug.
Linezolid is a recommended empiric therapy option for methicillin-resistant Staphylococcus aureus in hospitalized adult and pediatric patients with complicated skin and soft tissue infection, for methicillin-resistant Staphylococcus aureus-associated skin and soft tissue infection in the community, and Purulent and non-purulent cellulitis associated with methicillin-resistant Staphylococcus aureus [4]. Linezolid has been reported to be the first available oxazolidinone that inhibits bacterial protein synthesis by interfering with translation. Linezolid binds to a site on the bacterial 23S ribosomal RNA in the 50S subunit, preventing the formation of a functional 70S initiation complex. This is in order to prevent the bacteria from multiplying by inhibiting its protein production [5]. Linezolid is bactericidal against most strains of streptococci and bacteriostatic against staphylococci and enterococci; this makes linezolid a poor choice for immunosuppressed patients. Myelosuppression is a condition in which bone marrow activity decreases, resulting in fewer red blood cells, white blood cells, and platelets. Pancytopenia is a hematologic condition characterized by a decrease in all three peripheral blood cell lines. It is characterized by hemoglobin less than 12 g/dL in women and 13 g/dL in men, platelets less than 150,000 per mcL, and white blood cells less than 4,000 per mL (or absolute neutrophil count less than 1,800 per mL), [6].
Leukopenia is primarily seen as neutropenia since neutrophils make up the majority of leukocytes. Pancytopenia is not a disease but a manifestation of other underlying conditions [7]. So the importance of periodic evaluations and blood tests should be explained to a patient if they started medications such as methotrexate or linezolid [8]. Given that Linezolid is gaining great importance due to the resistance that some bacteria present to vancomycin, it is convenient to carry out this work, in order to provide updated and accurate information on the adverse and hematological effects of Linezolid and thus be able to identify its correctness. use as well as its forecast.
MATERIALS AND METHODS
A systematic review was carried out, the databases used were PubMed, Scielo and ScienceDirect. Articles indexed in Englishlanguage journals were chosen. In this review, 92 original and review publications related to the topic studied were identified, of which 25 articles met the specified inclusion requirements, such as being full-text articles and reporting on the topic. As exclusion criteria, it was taken into account that the articles did not have sufficient information and that they did not present the full text at the time of their review.
RESULTS
Linezolid and Myelosuppression
Linezolid is used as an effective antibiotic against bacteria belonging to the gram-positive group. This antibiotic has been associated with reversible myelosuppression. Myelosuppression has been reported to be dependent on the time and dose of instituting linezolid [9]. At high doses up to 1,000 mg/kg/day reversible myelosuppression occurred, and at low doses up to 10 to 40 mg/kg/day hematologic findings involved erythrocytes and reticulocytes, but mild effects on white blood cell and count were observed. of platelets [10].
Given linezolid’s efficacy for the treatment of severe grampositive infections, activity against resistant pathogens, and equivalent intravenous to oral formulations, the benefits of linezolid treatment may outweigh the potential risk of reversible myelosuppression [11]. It should be noted that pediatric patients are not excluded from the side effects of linezolid. Although antibiotic resistant infections constitute a significant part of serious childhood infections. In infections caused by enterococci, staphylococci, and pneumococci, a gradual increase in resistance and difficulty of treatment is observed. So, Linezolid is one of the new antibiotics that has recently been introduced for clinical use with gram positive efficacy [12]. Linezolid is the first introduced antibiotic of the oxazolidinone group. Linezolid was approved by the Food and Drug Administration for use in children. Linezolid stops bacterial growth by inhibiting protein synthesis. It is effective against microorganisms including vancomycin-resistant Enterococcus faecium and Enterococcus faecalis, methicillinresistant staphylococci, and penicillin-resistant pneumococci [13].
Linezolid and Central Nervous System Infections
In the absence of meningeal involvement, the level of linezolid in cerebrospinal fluid (CSF) is 70% of the plasma level. It is used in children because it is highly penetrating into the CSF and has few side effects [14]. It is preferred in the treatment of resistant gram-positive bacterial infections, especially in the treatment of vancomycin-resistant enterococcal infections in childhood [10]. Healthcare-associated central nervous system infections constitute 0.4% of all nosocomial infections. Health care-associated central nervous system infections have a high morbidity and mortality rate [9].
Risk factors include interventions directed at the brain, prolonged surgical time, intracerebral hemorrhage, CSF leak, and the presence of another source of infection in the body. The most common pathogens in healthcare-associated central nervous system infections include Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus spp., Pseudomonas aeruginosa, and E. coli. Over time resistance is gradually increasing among enterococci. Especially, the number of vancomycin-resistant pediatric cases is gradually increasing [15]. In comparative studies conducted with vancomycin, linezolid was found to be as effective and safe as vancomycin. Linezolid is the most appropriate treatment option in the treatment of infections caused by vancomycin-resistant enterococci [16]. The most important factor in nosocomial infections of the central nervous system is the selection of the appropriate antibiotic. Linezolid has been approved for use in the treatment of community-acquired and nosocomial pneumonia and skin and soft tissue infections. In Figure 1 we can identify the main indications for linezolid [17,18].
Other Adverse Reactions Caused by Linezolid
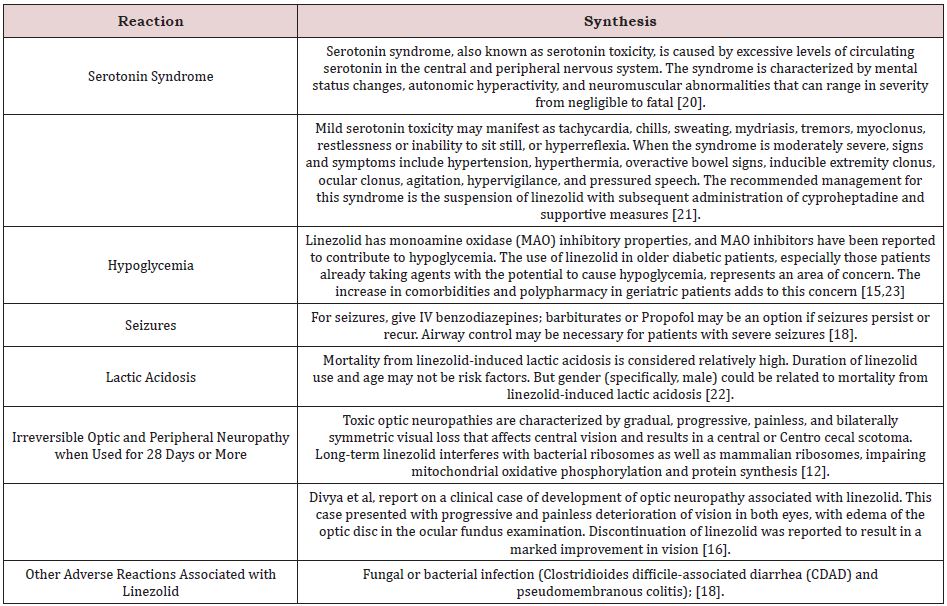
The main side effect associated with this drug is the decrease in platelets, hemoglobin and white blood cell counts, we can also find a series of non-specific but associated symptoms such as headache, nausea, diarrhea, elevated pancreatic enzymes, elevated liver function tests. and neuropathy. Table 1 shows other frequent reactions in patients who use the antibiotic linezolid for a long time and at a high dose [18-20].
Reaction Synthesis
Serotonin syndrome Serotonin syndrome, also known as serotonin toxicity, is caused by excessive levels of circulating serotonin in the central and peripheral nervous system. The syndrome is characterized by mental status changes, autonomic hyperactivity, and neuromuscular abnormalities that can range in severity from negligible to fatal [20]. Mild serotonin toxicity may manifest as tachycardia, chills, sweating, mydriasis, tremors, myoclonus, restlessness or inability to sit still, or hyperreflexia. When the syndrome is moderately severe, signs and symptoms include hypertension, hyperthermia, overactive bowel signs, inducible extremity clonus, ocular clonus, agitation, hypervigilance, and pressured speech. The recommended management for this syndrome is the suspension of linezolid with subsequent administration of cyproheptadine and supportive measures [21]. Hypoglycemia Linezolid has monoamine oxidase (MAO) inhibitory properties, and MAO inhibitors have been reported to contribute to hypoglycemia. The use of linezolid in older diabetic patients, especially those patients already taking agents with the potential to cause hypoglycemia, represents an area of concern. The increase in comorbidities and polypharmacy in geriatric patients adds to this concern [15,22].
Seizures for seizures, give IV benzodiazepines; barbiturates or Propofol may be an option if seizures persist or recur. Airway control may be necessary for patients with severe seizures. Lactic acidosis Mortality from linezolid-induced lactic acidosis is considered relatively high. Duration of linezolid use and age may not be risk factors. But gender (specifically, male) could be related to mortality from linezolid-induced lactic acidosis [23].
Irreversible optic and peripheral neuropathy when used for 28 days or more toxic optic neuropathies are characterized by gradual, progressive, painless, and bilaterally symmetric visual loss that affects central vision and results in a central or centro cecal scotoma. Long-term linezolid interferes with bacterial ribosomes as well as mammalian ribosomes, impairing mitochondrial oxidative phosphorylation and protein synthesis.
Divya et al. [22] report on a clinical case of development of optic neuropathy associated with linezolid. This case presented with progressive and painless deterioration of vision in both eyes, with edema of the optic disc in the ocular fundus examination. Discontinuation of linezolid was reported to result in a marked improvement in vision. Other adverse reactions associated with linezolid Fungal or bacterial infection (Clostridioides difficileassociated diarrhea (CDAD) and pseudomembranous colitis).
DISCUSSION
Linezolid is a new broad-spectrum oxazolidinone antibiotic that primarily targets Gram-positive bacteria, with particularly desirable coverage of increasingly prevalent resistant organisms, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. The most common side effects of this drug include gastrointestinal and hematological. Sanjana et al. [16] informs us of a case of a 72-year-old male patient, who was hospitalized with shortness of breath for 10 days, fatigue, and generalized weakness for seven days. The patient had undergone bilateral total knee replacement surgery for osteoarthritis of the knee two months prior to the current admission. At the orthopedic check-up one month after surgery, the patient complained of pain and swelling in the right knee. Culture of a synovial fluid aspirate from the affected knee revealed methicillin-resistant Staphylococcus aureus, sensitive to vancomycin and linezolid, for which she received linezolid. After four weeks of treatment, he went for a check-up, showing pancytopenia, for which this drug was discontinued. packed red blood cells were transfused and there was a significant improvement in dyspnea. The prescription of Linezolid will increase exponentially due to the ubiquity of these organisms. Therefore, linezolid prescriptions must be followed with due caution and control of any adverse event [24].
Another study carried out by Hideo et al, in which they carry out a systematic review of the effects of linezolid in pediatric patients, reaching the conclusion that the incidence of myelosuppression induced by linezolid in pediatric patients is less than 10%. Treatment duration of more than 14 days was also reported to be one of the risk factors associated with linezolid-induced myelosuppression. Therefore, careful follow-up is necessary in these patients [25]. There are many studies that affirm the hematological effects of Linezolid, as well as warn of the possible risks and precautions to take into account when implementing this drug, so it is necessary to establish a follow-up in all patients who use this drug. A strength of the current study is the methodology implemented, regarding the literature search, and steps in the selection of relevant articles, quality assessment, and data extraction. However, this study has several limitations, which should be taken into account before reaching a conclusion, among these are the few studies that explain or report the pathophysiological mechanisms by which linezolid produces myelosuppression, and thus be able to know and adequately treat these adverse effects, so more studies are needed to answer these questions.
CONCLUSION
Linezolid is used as an effective antibiotic against bacteria belonging to the gram-positive group. This antibiotic has been associated with reversible myelosuppression. At high doses up to 1,000 mg/kg/day reversible myelosuppression occurred, and at low doses up to 10 to 40 mg/kg/day hematologic findings involved erythrocytes and reticulocytes, but mild effects on white blood cell and count were observed. of platelets. In the absence of meningeal involvement, the level of linezolid in cerebrospinal fluid (CSF) is 70% of the plasma level. It is used in children because it is highly penetrating into the CSF and has few side effects. In comparative studies conducted with vancomycin, linezolid was found to be as effective and safe as vancomycin. This antibiotic also presents other adverse reactions to take into account such as serotonin syndrome, hypoglycemia, among other conditions reported in Table 1.
REFERENCES
- Azzouz A, Preuss C (2022) Linezolid. StatPearls Publishing, Florida, USA.
- Vargas-Carretero CJ, Fernandez-Vargas OE, Ron-Magaña AL, Padilla- Ortega JA, Ron-Guerrero CS, et al. (2019) Etiology and clinicohematological profile of pancytopenia: experience of a Mexican Tertiary Care Center and review of the literature. Hematology 24(1): 399-404.
- Das Makheja K, Kumar Maheshwari B, Arain S, Kumar S, Kumari S, et al. (2013) The common causes leading to pancytopenia in patients presenting to tertiary care hospital. Pak J Med Sci 29(5): 1108-1111.
- Jain A, Naniwadekar M (2013) An etiological reappraisal of pancytopenia - largest series reported to date from a single tertiary care teaching hospital. BMC Hematol 13(1): 10.
- Gnanaraj J, Parnes A, Francis CW, Go RS, Takemoto CM, et al. (2018) Approach to pancytopenia: Diagnostic algorithm for clinical hematologists. Blood Rev 32(5): 361-367.
- Takeshima M, Ishikawa H, Kitadate A, Sasaki R, Kobayashi T, et al. (2018) Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry 18(1): 150.
- Martín Pozuelo Ruiz de Pascual R, López Pardo P, López-Dóriga Bonnardeaux P (2020) Pancytopenia during SARS-CoV-2 infection. Med Clin 155(8): 364-365.
- Zhao Y, He J, Wang J, Li WM, Xu M, et al. (2021) Development of pancytopenia in a patient with COVID-19. J Med Virol 93(3): 1219-1220.
- Bagheri Z, Labbani-Motlagh Z, Mirjalili M, Karimzadeh I, Khalili H (2020) Types and outcomes of cytopenia in critically ill patients. J Comp Eff Res 9(9): 627-637.
- Xiao N, Hao S, Zhang Y, Shao Z (2020) Roles of immune responses in the pathogenesis of immunorelated pancytopenia. Scand J Immunol 92(2): e12911.
- Garla VV, Abdul Salim S, Yanes-Cardozo LL (2018) Pancytopenia: a rare complication of Graves’ disease. BMJ Case Rep 2018.
- Hamid OA, Fadul AM, Batia TB, Yassin MA (2020) Graves’ disease-related pancytopenia improved after radioactive iodine ablation. Case Rep Oncol 13(1): 295-298.
- Opie J, Omar F, Huang HC, Sandler L, Ross I (2017) Primary hyperparathyroidism manifesting with pancytopenia. Pathology 49(3): 326-329.
- Achi HV, Ahui BJ, Anon JC, Kouassi BA, Dje-Bi H, et al. (2013) Pancytopenia: a severe complication of miliary tuberculosis. Rev Mal Respir 30(1): 33-37.
- Santiago-Rodríguez EJ, Mayor AM, Fernández-Santos DM, Hunter- Mellado RF (2015) Profile of HIV-Infected Hispanics with Pancytopenia. Int J Environ Res Public Health 13(1): 13010038.
- Tveiten H, Aukrust P, Lehne G, Rodriguez JR, Skjønsberg OH (2020) Haemophagocytic lymphohistiocytosis in COVID-19 cases? Tidsskr Nor Laegeforen 140(6).
- Mutschler M, Trojan S, Defosse JM (2013) Severe sepsis caused by a linezolid-resistant Enterococcus faecium in a 10-year-old girl after multiple trauma. Int J Infect Dis 17(6): e466-e467.
- Yılmaz A, Dalgıc N, Musluman M, Sancar M, Colak I, et al. (2010) Linezolid treatment of shunt-related cerebrospinal fluid infections in children. J Neurosurg Pediatr 5: 443-448.
- Ager S, Gould K (2012) Clinical update on linezolid in the treatment of gram-positive bacterial infections. Infect Drug Resist 5: 87-102.
- Parlak E, Tan H (2015) Pancytopenia due to linezolid treatment. Turk Pediatri Ars 50(3): 185-188.
- Shouan A, Kumar R, Lal V, Grover S (2020) Linezolid-induced serotonin syndrome. Ind Psychiatry J 29(2): 345-348.
- Mao Y, Dai D, Jin H, Wang Y (2018) The risk factors of linezolid-induced lactic acidosis. Medicine (Baltimore) 97(36): e12114.
- Johannesmeyer H, Bhakta S, Morales F (2017) Linezolid-Associated Hypoglycemia. Drug Saf Case Rep 4: 18.
- Sharma S, Syal A, Gupta M, Tahlan A, Kaur B (2020) Reversible myelosuppresion with prolonged usage of linezolid in treatment of methicillin-resistant Staphylococcus aureus. Cureus 12(10): e10890.
- Kato H, Hagihara M, Asai N, Koizumi Y, Yamagishi Y, et al. (2021) A systematic review and meta-analysis of myelosuppression in pediatric patients treated with linezolid for Gram-positive bacterial infections. J Infect Chemother 27(8): 1143-1150.
Article Type
Research Article
Publication history
Received Date: December 06, 2022
Published: February 16, 2023
Address for correspondence
Jhon Edison Meneses Sanchez, General physician, Universidad Surcolombiana, Neiva-Huila, Colombia; https://orcid.org/0000-0003- 1937-5294
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Jhon Edison MS, Juan Pablo GM, Francisco Alberto RL, Eliana Rocio AF, Luis Alberto GZ, et. al. Myelosuppression and Other Adverse Reactions Caused by Linezolid. 2023- 5(1) OAJBS.ID.000545.