Histopathologic Finding and Pathophysiology Pathway of Moluskum Kontangiosum
ABSTRACT
Introduction: Molluscum contagiosum is endemic in densely populated communities, poor hygiene, and poor areas. This
disease mainly affects children, adults with active sexual activity and immunodeficiency status. Transmission can be through direct
contact with active lesions or autoinoculation, indirect transmission through sharing personal tools such as towels, razors, hair
clippers and transmission through sexual contact. The prevalence of Molluscum contagiosum in the world varies. In the US it is
33%, in Mali 3.6%, in Australia the overall seropositivity rate is 23%, in East Africa it is 52% in children aged 2 years. The incidence
of molluscum contagiosum worldwide is estimated at 2% - 8%, with a prevalence of 5% - 18% in HIV/AIDS patients. Molluscum
contagiosum virus type-1 (MCV-1) was the most common subtype found in patients, whereas MCV-3 was rare. For example, analysis
of 106 clinically isolated MCVs indicated the presence of MCV-1, -2, and -3 in an 80:25:1 ratio. In addition, MCV-2 was found to be
more common in adults.
Discussion: Cystic changes in superficial MCV lesions may occur. There is a small ostium opening to the skin surface, which
potentially connects the MCV and facilitates the extension of the MCV from the infected EIC to the adjacent skin. Several poxviruses
can determine persistent infection in cell culture. The rate of infection in adults with AIDS raises the possibility of reactivation of
subclinical infection in the setting of immunosuppression. While genital lesions are found in sexually active adults.Histopathology
of molluscum contagiosum shows a proliferation of stratum spinosum cells that form lobules with central cellular and viral debris.
The intraepidermal lobules are separated by connective tissue septa and the molluscum bodies in the lobules are found in the form
of round or oval cells that undergo keratohyaline degeneration. In the stratum basalis, there is a picture of cell mitosis with enlarged
basophilic nuclei. In the advanced phase, cells that undergo a cytoplasmic vacuolization process can be found and eosinophilic
globies are obtained. In several cases of molluscum contagiosum lesions with secondary infection, the predominant inflammatory
picture of lymphocytes and neutrophils was found on histopathological examination.
Conclusion: Widespread involvement of eczematous areas has been described in patients with atopic dermatitis and is
associated with skin disorders, use of topical steroids, and/or underlying disorders. Patients with HIV infection have a marked
increase in infection. It was first noted in the early years of the AIDS epidemic that molluscum contagiosum in HIV-infected
people was recognized as a common opportunistic infection. Both prevalence and severity of disease increase with increasing
immunodeficiency, with lesions increasing in up to one third of patients with CD4 cell counts of 100 cells/mm or below.
INTRODUCTION
Molluscum contagiosum is endemic in densely populated communities, poor hygiene and poor areas. This disease mainly affects children, adults with active sexual activity and immunodeficiency status. Transmission can be through direct contact with active lesions or autoinoculation, indirect transmission through sharing personal tools such as towels, razors, hair clippers and transmission through sexual contact. The prevalence of Molluscum contagiosum in the world varies. In the US it is 33%, in Mali 3.6%, in Australia the overall seropositivity rate is 23%, in East Africa it is 52% in children aged 2 years. The incidence of molluscum contagiosum worldwide is estimated at 2% - 8%, with a prevalence of 5% - 18% in HIV/AIDS patients [1].
Molluscum Contagiosum Virus (MCV) is a weak immunogen. About a third of patients do not produce antibodies to MCV, so frequent attacks are repeated. Three subtypes of MCV have been identified, all of which have a similar clinical presentation and are not localized to a specific body part (eg genital). Molluscum contagiosum virus type-1 (MCV-1) was the most common subtype found in patients, whereas MCV-3 was rare. For example, analysis of 106 clinically isolated MCVs indicated the presence of MCV-1, -2, and -3 in an 80:25:1 ratio. In addition, MCV-2 was found to be more common in adults [1,2].
In studies in Indian and Alaska, states that children under 15 years are more often exposed to molluscum contagiosum. Transmission can occur through direct skin contact or sexual intercourse. It is often affected in children, as well as in adults, although in adults it is usually affected at the genital site due to sexual transmission. In this molluscum spread can be through autoinoculation, so when the patient scratches or shaves the lesions will slowly spread to other parts of the body. Transmission can be through direct contact with active lesions or autoinoculation, indirect transmission through sharing personal tools such as towels, razors, hair clippers and transmission through sexual contact. Infection has been associated with procedures causing skin trauma (eg, shaving, tattooing, and electrolysis) and with contact with fomites, such as baths, gymnastic equipment and towels, and especially swimming pools, which have contributed to outbreaks in society [1,3].
Transmission of MCV occurs primarily by skin-to-skin contact by both sexual and non-sexual routes and may be enhanced by conditions of warmth and humidity, with infection more common in tropical areas. Location of lesions (eg, genitals and pubic skin), history of frequent contact with multiple sexual partners and prostitutes, history and presence of other STDs, presence of genital lesions in sexual partners, and age of peak occurrence (20-29 years) that is similar to STD are believed Caused by sexual transmission [2-4].
DISCUSSION
MCV has the most limited range of tissue tropism properties than poxviruses. Infection occurs only in the epidermis, and spread does not occur deeply even in immunocompromised patients. MCV has a predilection for the hair follicle epithelium so that it is rarely found in non-hair follicle areas such as the palms of the hands, soles of the feet, and mucosa. Coexistence of MCV infection in Epidermal inclusion cyst (EIC) or usually by external autoinoculation. The general variety of superficial MCV lesions may exhibit some degree of multilocularity [5].
Pathophysiology Pathway
Cystic changes in superficial MCV lesions may occur. There is a small ostium opening to the skin surface, which potentially connects the MCV and facilitates the extension of the MCV from the infected EIC to the adjacent skin. Several poxviruses can determine persistent infection in cell culture. The rate of infection in adults with AIDS raises the possibility of reactivation of subclinical infection in the setting of immunosuppression. While genital lesions are found in sexually active adults [6].
A study shows evidence of a rapid immunological response as a mechanism resolution of the inflammatory lesion. Meanwhile, not all involved MC lesions had pre-existing clinical inflammation. The general pattern of MCV growth mimics the pattern of follicular neogenesis in which the pale lobular palisade basal cells resemble those of the hair follicle root cells and central punctum. MCV lesions can simulate the ostium of a hair follicle. As a result, there is a holocrine secretion of MC bodies to the surface, which can produce immune modulators and viral affinity to infect the follicular epithelium. The infection originates in the hair follicles where the virus will induce the release of growth factors leading to rapid epidermal proliferation [1,7].
Cells produce images similar to the anagen phase of hair follicles, then the growth phase ends with the involution and degeneration of keratinocytes. Molluscum contagiosum exhibits characteristic cup-shaped endophytic lobules of acanthoti squamous epithelium. Eosinophilic intracytoplasmic inclusions accumulate and enlarge until they replace the entire cell. Then it is extruded on the surface through the ostium as formed by the central creater. Many lesions are not inflamed, but some exhibit a dense reddish lymphocytic infiltrate [8].
The pathological changes caused by MCV infection are very characteristic. The lesion consisted primarily of a hyperplastic epidermis surrounding lobules filled with keratin debris and degenerating molluscum bodies. In the basal layer, the nucleus and cytoplasm of the keratinocytes are enlarged and there is an increase in mitosis.
In the spinosum layer, as a result of viral replication in the cytoplasm, the cells begin to display cytoplasmic vacuolization, enlargement, and are then replaced with eosinophil globules, molluscum bodies, contained in well-defined sacs and suppression of the nucleus for peripheral cells. In the granular layer, the molluscum bodies become more homogeneous with loss of internal parts and structural markings and finally desquamated to cystic lobule changes. The dermis is usually limited to stromal proliferation, although inflammation occurs in up to 20% of clinical lesions, with necrotic epithelial infiltration by lymphocytes, histiocytes, neutrophils, and occasionally nucleated cells. In HIV infection, histologic lesions may be atypical, with hyperkeratosis and verrucous changes [9].
Histopathological Findings
Lesions are rarely found on the palms and soles. Sometimes it enlarges by 10-15 mm, producing a “giant molluscum.” smooth, dome-shaped flesh-colored papules, with characteristic central umbilication. In adults, the lesions most commonly occur on the thighs, inguinal region, buttocks, and lower abdominal wall and less commonly on the external genitalia and perianal area. The pattern differs with the distribution of genital warts. Children more commonly develop lesions on the face, trunk, and upper extremities, often with a linear distribution indicating autoinoculation with scratching; lesions on the palms, soles, and mucous membranes are rare [10].
In adults, lesions can also be found in the perigenital and perianal areas. Lesions are usually more extensive in children than in adults, and while adults with venereal disease rarely develop extragenital lesions, 10–50% of infected children have genital lesions. The median duration of untreated disease was reported to be approximately 2 years, ranging from 2 weeks to 4 years; Individual lesions usually disappear within 2 months. Recurrence occurs in 15-35% of patients, usually with complications [11].
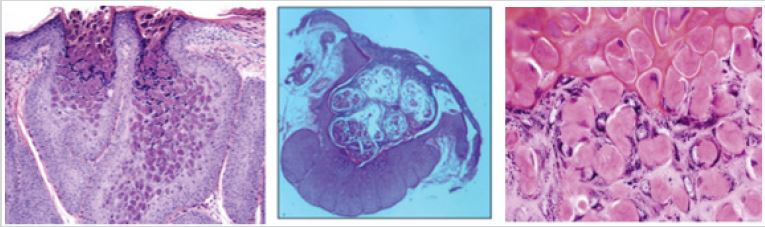
Histopathology of molluscum contagiosum shows a proliferation of stratum spinosum cells that form lobules with central cellular and viral debris. The intraepidermal lobules are separated by connective tissue septa and the molluscum bodies in the lobules are found in the form of round or oval cells that undergo keratohyaline degeneration. In the stratum basalis, there is a picture of cell mitosis with enlarged basophilic nuclei. In the advanced phase, cells that undergo a cytoplasmic vacuolization process can be found and eosinophilic globies are obtained (Figure 1). In several cases of molluscum contagiosum lesions with secondary infection, the predominant inflammatory picture of lymphocytes and neutrophils was found on histopathological examination [11,12].
The importance of the immune system in controlling MCV. In the epidermis, the inflammatory infiltrate is usually minimal. The body’s defense is very important to control the virus, the virus will be isolated at the top of the epidermis. Epidermal inflammation This minimal inflammation occurs usually after trauma. In addition, the greater prevalence of lesions in children than in adults indicates the acquisition of host resistance to age. The existence of values indicating the severity of infection in patients with AIDS suggests that cell-mediated immunity (CMI) is important in the control of MCV [10].
Clinical Manifestations
The clinical and histopathological changes of molluscum contagiosum are identical to those seen in horses with Uasin Gishu disease. However, the causative agent equine molluscum contagiosum cannot grow in culture, whereas the agent Uasin Gishu can be cultured. Lesions usually begin in one area, such as the chest, shoulder, neck, or limb, then spread, with some affected animals having hundreds of lesions. Occasionally, MCV lesions may remain localized in areas such as the snout, axilla, inguinal region, or genitalia. The lesions begin as papules that become hyperplastic with thick crusts and horny projections. Papules may be umbilicated with a central pore, often containing a caseous plug [13].
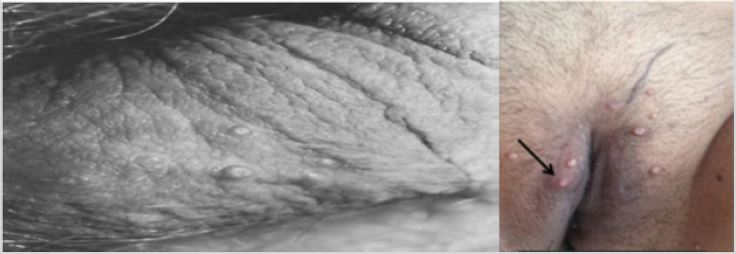
The incubation period is 2 weeks to 6 months, and individual lesions last 2 months. The entire development may take 9 months to 2 years or more. Individual lesions are papules that are fleshy or pale pink in color. Its characteristic is to have a center, ranging in size from 1 to 5 mm, can occur in any part of the body. Lesions tend to cluster in one or two areas, especially skin folds (axillary, neck, inguinal folds). The number of lesions is usually less than 20 lesions. The number can increase to hundreds, especially in immunocompromised patients. Pruritus or surrounding dermatitis may occur, and occasionally, the lesions become inflamed and bleed (Figure 2). In advanced acquired immunodeficiency syndrome, when the lesions are numerous on the face and scalp. Progression can be conjunctivitis if lesions are present around the lids [14].
The most frequent complication of infection, “molluscum dermatitis”, appears 1-15 months after the onset of the lesion in up to 10% of patients. Consists of a well-defined, eczematoid reaction 3-10 cm in diameter with approximately a single lesion, may involve only part of the lesion, and usually disappears as the lesion heals. Eyelid lesions may induce unilateral conjunctivitis. This pattern is usually that of chronic follicular or papillary conjunctivitis, but corneal changes with epithelial keratitis similar to trachoma may also occur. Other inflammatory conditions in association with molluscum contagiosum include folliculitis, sycosis barbae, erythema annular centrifugum, and pseudoleukemia cutis. Infection does not appear to affect pregnancy outcome, and while lesions have been reported in infants up to 1 week of age, there have been no documented cases of maternal-fetal transmission [15,16].
CONCLUSION
Widespread involvement of eczematous areas has been described in patients with atopic dermatitis and is associated with skin disorders, use of topical steroids, and/or underlying disorders. Patients with HIV infection have a marked increase in infection. It was first noted in the early years of the AIDS epidemic that molluscum contagiosum in HIV-infected people was recognized as a common opportunistic infection. Both prevalence and severity of disease increase with increasing immunodeficiency, with lesions increasing in up to one third of patients with CD4 cell counts of 100 cells/mm or below.
REFERENCES
- Olsen JR, Piguet V, Gallacher J, Francis NA (2016) Molluscum contagiosum and associations with atopic eczema in children: A retrospective longitudinal study in primary care. Br J Gen Pract 66(642): e53-e58.
- Haque M, Coury DL (2018) Treatment of molluscum contagiosum with an East Indian sandalwood oil product. Journal of Dermatologic Treatment 29(5): 531-533.
- Silverberg NB (2018) Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis 102(3): 191-194.
- Kaufman WS, Ahn CS, Huang WW (2018) Molluscum contagiosum in immunocompromised patients: AIDS presenting as molluscum contagiosum in a patient with psoriasis on biologic therapy. Cutis 101(2): 136-140.
- Haddock ES, Cheng CE, Barrio VR (2017) Extensive orf infection in a toddler with associated id reaction. Pediatric Dermatology 36(6): e337-e340.
- Manti S, Amorini M, Cuppari C, Annamaria S, Francesca P, et al. (2017) Filaggrin mutations and Molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol 119(5): 446-451.
- Fisher C, McLawhorn JM, Adotama P, Stasko T, Collins L, et al. (2019) Pulsed dye laser repurposed: Treatment of refractory molluscum contagiosumin renal transplant patient. Transplant Infectious Disease 21(2): e13036.
- Jahnke MN, Hwang S, Griffith JL, Shwayder T (2018) Cantharidin for treatment of facial molluscum contagiosum: A retrospective review. Journal of the American Academy of Dermatology 78(1): 198-200.
- Berger EM, Orlow SJ, Patel RR, Schaffer JV (2012) Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: The bump that rashes. Arch Dermatol 148(11): 1257-1264.
- DiBiagio JR, Pyle T, Green JJ (2018) Reviewing the use of imiquimod for molluscum contagiosum. Dermatology Online Journal 24(6): 13030.
- Hayashida S, Furusho N, Uchi H, Shougo M, Kunimitsu E, et al. (2010) Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci 60(3): 173-178.
- Giner-Soriano M, Teixidó C, Marsal JR, Diez O, Pera H, et al. (2019) Randomized placebo-controlled clinical trial on efficacy and safety of topical 10% potassium hydroxide for molluscum contagiosum treatment in children. Journal of Dermatological Treatment 30(8): 750-756.
- Go U, Nishimura-Yagi M, Miyata K, Mitsuishi T (2018) Efficacy of combination therapies of topical 5% imiquimod and liquid nitrogen for penile molluscum contagiosum. Journal of Dermatology 45(10): e268-e269.
- Schaffer JV, Berger EM (2016) Molluscum contagiosum. JAMA Dermatol 152(9): 1072.
- Fornatora ML, Reich RF, Gray RG, Freedman PD (2001) Intraoral molluscum contagiosum: A report of a case and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92(3): 318- 320.
- Brown J, Janniger CK, Schwartz RA, Silverberg NB (2006) Childhood molluscum contagiosum. Int J Dermatol 45(2): 93-99.
Article Type
Review Article
Publication history
Received Date: December 23, 2021
Published: January 06, 2022
Address for correspondence
Nanda Rachmad Putra Gofur, Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Indonesia
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Nanda RPG, Aisyah RPG, Soesilaningtyas, Rizki NRPG, Mega K, Hernalia MP. Histopathologic Finding and Pathophysiology Pathway of Moluskum Kontangiosum. 2022- 4(1) OAJBS.ID.000373.