Gene-to-Face
ABSTRACT
Malocclusion results from the interaction of genetic and environmental factors on the development of the orofacial region. Malocclusion associated with genetic syndrome often requires multidisciplinary approach. With the advent of gene therapy, efforts are made to correct the defective genes. Hence it is important understand the role of various genes in the development of craniofacial growth. An understanding of these genes causing abnormalities is necessary for the dentist to make the appropriate referrals to ensure that the patient receives the best available care.
KEYWORDS
Genetics; Genes; Craniofacial Growth; Malocclusion
ABBREVIATIONS
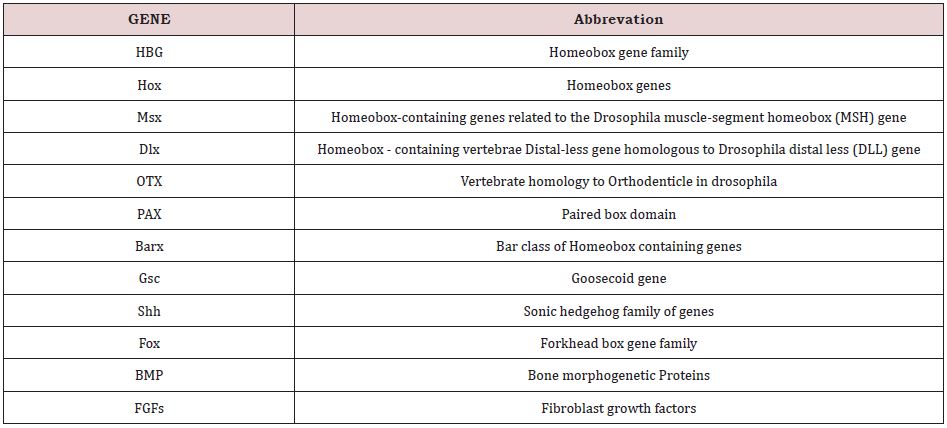
HBG: Homeobox gene family; Hox: Homeobox genes; Msx: Homeobox-containing genes related to the Drosophila muscle-segment homeobox (MSH) gene; Dlx: Homeobox - containing vertebrae Distal-less gene homologous to Drosophila distal less (DLL) gene; OTX: Vertebrate homology to Orthodenticle in drosophila; PAX: Paired box domain; Barx: Bar class of Homeobox containing genes; Gsc: Goosecoid gene; Shh: Sonic hedgehog family of genes; Fox: Forkhead box gene family; BMP: Bone morphogenetic Proteins; FGFs: Fibroblast growth factors
INTRODUCTION
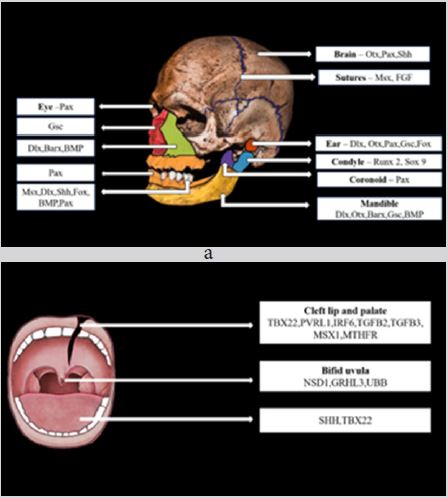
Growth occurs as a result of interaction between several genetic and environmental factors. It has become essential nowadays to understand the role of genes in growth and also in various diseases and syndromes to establish appropriate treatment planning. Malocclusion results from the interaction of genetic and environmental factors on the development of the orofacial region. Malocclusion associated with genetic syndromes is difficult to manage clinically and often requires a multidisciplinary approach. Various genes involved in craniofacial growth is depicted in Figure 1; Table 1. With the advent of gene therapy many experiments have been conducted by researchers on animals to correct the defective genes associated with particular conditions. In orthodontics, research have been done on gene therapy for orthodontic tooth movement and pain control during tooth movement. Various findings and development in the field of molecular genetics have rendered valuable information related to craniofacial growth.
Genetic factors play an important role in the development of malocclusion. Lauweryns in 1993 stated that 40% of dental and skeletal malocclusions are attributed to genetic factors. A study on triplets by Kraus, Wise, and Friel showed that the morphology of individual bone is under strong genetic control. Various studies such as Twin studies, Pedigree studies, and Inbreeding aids in highlighting the role of environmental and genetic causes on particular behaviour or disease. EDA (Ectodysplasin A) and XEDAR (X- linked Ectodermal dysplasia Receptor gene) are suggested to be associated with Class I dental crowding patients. The EDA signalling pathway also plays a morphogenetic role in teeth and ectodermal organs. The concept of polygenic inheritance for class II division I malocclusion is suggested by cephalometric studies by Harris. However, environmental factors also contribute to the etiology of Class II division I malocclusion. In the case of class II division 2 malocclusion Twin studies showed a 100% concordance rate, indicating a strong genetic influence in the development of class II division II deep bite malocclusion. PAX 9 and RUNX 2 genes are also found to have some contribution in the development of class II division II malocclusion. The influence of genetic factors in Class III malocclusion (Hapsburg jaw) can be monogenic in some cases and polygenic in others.
HBG (HOMEOBOX GENE FAMILY)
The genes of this family code for homeodomain, a protein that combines with DNA and modulates the expression of various genes. These genes are involved in embryonic patterning. The study conducted in mice shows that a defect in Hox a-2 gene caused the absence of derivatives of the second branchial arch and also the change of second pharyngeal arch structures to first-arch structures. For instance, in the absence of stapes, supernumerary malleus had developed.
Besides the Hox-cluster genes homeodomain is present in numerous other transcription factors. For instance, Tlx and Dlx genes are involved in the patterning of structures in the brain and craniofacial region. Mutation of the Pax 3 gene is seen in Waardenburg syndrome, a genetic syndrome associated with hearing loss and change in pigmentation of hair, eyes, and skin. Mutation of homeodomain in the Msx-2 gene is seen in Boston-type craniosynostosis [1].
MSX HOMOEBOX GENE
This gene comes under the homeobox-containing superfamily. This gene is homologous to the Drosophila muscle segment homeobox gene. The MSX gene consists of 3 members namely Msx 1, Msx 2, and Msx 3. During embryogenesis, Msx 1 and Msx 2 are expressed widely in various organs, whereas Msx 3 is expressed only in the dorsal neural tube. In developing craniofacial regions, Msx 1 and Msx 2 are strongly expressed in an overlapping manner, indicating a role of Msx genes in craniofacial development [2,3]. Msx 2 deficient mice show defective skull ossification, indicating the role of Msx 1 and Msx 2 in the ossification of the skull. During embryonic development, Msx genes are also found to be expressed in the mesenchyme of cranial sutures and dura mater, but after birth, the expression of Msx 2 in sutural mesenchyme and duramater is reduced gradually [2,3].
Msx genes are expressed during tooth morphogenesis. During the bud stage, Msx 1 is evident in dental mesenchyme and Msx 2 in both epithelium and mesenchyme. In the cap stage, Msx 1 is expressed both in the dental follicle and dental papilla and Msx 2 in the enamel organ and inner enamel epithelium. Msx genes do not have a role in root morphogenesis in developing teeth. During the bell stage, the expression of Msx 2 is reduced in inner enamel epithelium but Msx 2 is strongly expressed in odontoblastic and sub odontoblastic regions. Msx 1 gene expression is seen in palatal mesenchyme and Msx 2 is detectable in the mandibular process and maxillary process [3].
Transgenic mice with non-functional Msx 1 causes cleft palate and complete anodontia [2].Missense mutation of Msx 1 causes selective tooth agenesis. Wolf-Hirschhorn syndrome, a congenital syndrome characterized by distinct facial characteristics and intellectual disability. Nonsense mutation of Msx 1 results in Witkop syndrome characterized by tooth agenesis and nail dysgenesis [3]. Msx 1 and Msx 2 are expressed in the mesenchyme of the medial region of the mandibular arch [4]. The medial part of the mandible is truncated in Msx 1 deficient mice [2].
DLX HOMEOBOX GENES
DLX is a homeobox-containing gene analogous to the Distalless gene (Dll) of Drosophila. This group of genes is found to be important in the embryonic patterning of limbs. This family of genes expressed in the embryonic ectoderm, the genital tubercle, developing teeth, and neural crest cells. They are also expressed during the morphogenesis of sense organs. Dlx genes play an important role in proximo-distal patterning of branchial arches [2]. Dlx-1 and Dlx-2 are expressed both in the maxillary and mandibular processes. On the other hand, Dlx-3, Dlx-6, and Dlx-5 are expressed only in the mandibular process [5]. Expression of Dlx 5 and Dlx 6 has been observed in osteoblasts of developing bones and in cartilages. Lack of Dlx 5 results in hypomineralization of the calvaria. Mutation of Dlx genes is associated with Tricho-dento-osseous syndrome, ectodermal dysplasias, and so on [2].
OTX GENES
Otx genes are homologous of drosophila genes coding for transcription factors. These genes play a crucial role in craniofacial development and brain morphogenesis particularly in the anterior region of the brain. Mutation of the OTX 2 gene causes various craniofacial anomalies, including mandibular hypoplasia and even absence of mandible in some cases. Loss of function of the same showed lack of development of the anterior head and decreased the survival rate of developing embryo. OTX 2 duplication is linked with Hemifacial microsomia and the risk of medulloblastoma in HFM is increased with OTX 2 duplication [6]. OTX 1-/- mutation in mice showed a decrease in the weight of the brain and epileptic behavior [7]. In Otx 2 +/- mice, elements derived from the midbrain crest are severely affected, but the hindbrain-derived elements are almost unaffected [2]. Otx 1 is also found to have a role in sense organ development.
PAX GENES
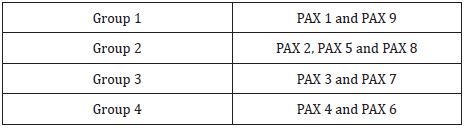
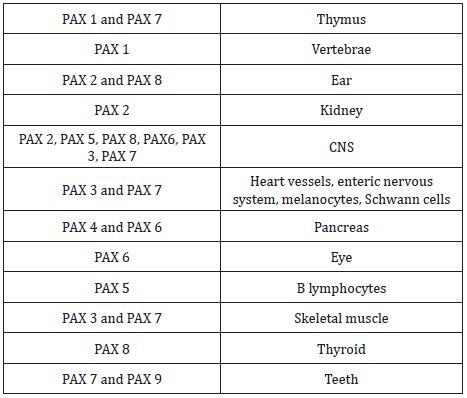
Pax genes (paired box gene) encodes for transcription factors that contain paired domain, a DNA binding domain that is the principal regulator of gene expression [8]. This family of genes consists of 9 members that are grouped into four groups showed in Table 2 [2]. Expression of group 1, group 3, and group 4 are seen in developing facial processes are shown in Table 3. An important role is played by PAX 3, PAX 6, and PAX 7 in the development of structures derived from neural crest cells in the upper face and PAX 9 for the lower face as shown in Table 3. In PAX 9 null mutants, structures derived from pharyngeal pouches are absent. Teeth development, alveolar ridge, and coronoid process are also affected in PAX 9 null mutants [9]. In dental mesenchyme, PAX 9 gene expression is controlled by BMP and FGF [9].
BARX GENES
BARX 1 is a protein (BARX homeobox 1) coding gene. In mouse and chick, the protein encoded by BARX 1 gene plays an important role in teeth development and are also expressed in craniofacial mesenchyme [10]. Epithelial-mesenchymal interactions are essential for the growth of facial primordia and studies conducted in mice show the expression of BARX 1 in the mesenchyme and BARX 2 in the epithelium. However, in chick embryos, BARX 1 is expressed both in epithelium and mesenchyme of developing maxillary and mandibular process [2]. In maxillary primordia, BARX 1 is expressed along the mediolateral axis. The expression of BARX-1 in the mesenchyme is controlled by BMP-4 and FGF- 8. Epithelial FGF-8 induces the expression of BARX-1 in the underlying mesenchyme and BMP-4 antagonize this induction of BARX-1 by FGF-8. Apart from developing facial primordia, BARX-1 is also expressed in developing stomach and appendicular skeleton (developing cartilage elements of limbs) [9].
GSC Gene
The goosecoid gene encodes a homeodomain-containing protein that acts as a transcription factor. This protein has been suggested to be involved in the organization of human embryos and also studies conducted in mice shows that it plays an essential role in craniofacial and ribcage development during embryogenesis [11,12]. GSC mutants cause various skeletal malformations including short stature, aural atresia, underdeveloped mandible. Mice that are homozygous for GSC gene mutation do not survive more than 24 hours. GSC -/- mice show hypoplasia of the lower jaw with a groove along with Meckel’s cartilage due to impaired interaction between mandibular cells and those of Meckel’s cartilage. Muscles associated with structures are also affected including tongue muscles. These phenotypes affect normal tongue movements causing impaired suckling and occlusion of airways causing neonatal death. Nasal cavity (aplastic nasal cavity and lack the ethmoid-derived turbinals), nasal pits, components of the inner ear, and external auditory meatus are also affected in GSC- /- mice [12]. GSC is highly expressed in the early stages of cancer progression and high expression of GSC indicates poor prognosis [11].
Shh gene
Sonic hedgehog gene encodes the protein Sonic hedgehog. It acts as a morphogenic signalling molecule that plays role in cell growth and organization of body plan. It is involved in patterning various systems including the anterior face, skull, lungs, and teeth. Apart from this, hedgehog signalling is also found to have a role in the development of limbs and digits. Hedgehog signalling plays an important role in patterning anterior neurocranium. Mutation of the Shh gene results in various malformations in the embryo such as Microphthalmia and Holoprosencephaly (the forebrain of the embryo fails to develop into two hemispheres with malformations in the face) [13]. Hedgehog signalling is also found to be regulating the development of stomodeum, or oral ectoderm [14]. In tooth development, SHH is released from the primary enamel knot that provides positional information of developing teeth and also regulated cusp growth. Mutation of Shh gene causes abnormal development of incisors and molars.
FOX GENE
Fox gene encodes Forkhead domain transcription factors (Fox proteins). Fox genes (Foxc-1, Foxc-2, Foxd-1, Foxd-2, Foxf-1, and Foxf-2) are found to be the major mediators of the function of Hh signalling in craniofacial morphogenesis [2]. Fox-C and Fox-F plays role in palate and bone formation. Foxc-1 has been linked to Axenfeld-Rieger syndrome (craniofacial and ocular defects). Mutant phenotype of Foxc-2 results in reduced upper facial cartilages with the absence of palatal components and middle ear ossicles. Foxf -1 and Foxf-2 mutants have cleft palate [2,15]. Foxf-1 and Foxf-2 are expressed in developing tooth buds. Foxf-1; Foxf-2a; Foxf-2b triple mutants show a complete absence of tooth buds [15].
BMP
BMP signalling is involved in the migration of neural crest cells so it is considered to be essential for embryonic and postnatal craniofacial growth and development. It regulates the growth of the maxilla, mandible, palate, and teeth. Growth of mandible is affected in BMP 7 deficient mice causing micrognathia. Apart from osteogenesis and myogenesis, BMPs also plays role in tooth development and mineralization.BMP 2, BMP 4, BMP 7 are expressed in primary enamel knot and also increase the expression of protein Ameloblastin that is associated with enamel mineralization [16]. Studies conducted in mouse shows that, among BMPs, BMP2, BMP3, BMP4 plays important role in the growth and development of the palate, and expression of BMP 2 is found both in anterior and posterior palate whereas BMP 3 is expressed only in the posterior palate [17].
FGF
Fibroblast growth factor receptors belong to the tyrosine kinase receptor family. They are expressed during calvarial bone development and are detected in developing bones and sutures.FGF- 2 is associated with osteoblastic differentiation and is expressed in cranial sutures and is weakly expressed in calvarial bones. On the other hand, FGF-9 is expressed in Dural layers and calvarial mesenchyme [2]. FGF-9 also plays important role in endochondral and intramembranous ossification and has an inhibitory role in osteogenic differentiation of bone marrow stem cells and dental pulp stem cells in in-vitro [18]. Mutation of FGFR causes various craniosynostosis syndromes that include Apert syndrome (FGFR- 2), Crouzon syndrome (FGFR-2 and FGFR-3), and Pfeiffer syndrome (FGFR-1 and FGFR-2). FGF is also expressed in dental epithelium and mesenchyme during tooth development.FGF-8, FGF-9, and FGF- 17 are expressed during the initiation of tooth development [19].
CONCLUSION
Genetic factors play an important role in development of malocclusion. Lauweryns in 1993 stated that 40% of dental and skeletal malocclusions are attributed to genetic factors. The pattern of growth and development is typically the result of an interaction between multiple genetic and environmental factors. Thus, the malocclusion seen in most of the instances is of polygenic/ multifactorial cause. Consideration of genetic factors plays essential part in orthodontic diagnosis and treatment planning of various malocclusion. In recent days it has been observed that malocclusion of genetic origin when treated during early stages of growth provides better outcome. An understanding of these genes causing abnormalities is necessary for the dentist to make the appropriate referrals to ensure that the patient receives the best available care. The orthodontist can be an integral part of the multidisciplinary team.
REFERENCES
- Thesleff I (1995) Homeobox genes and growth factors in regulation of craniofacial and tooth morphogenesis. Acta Odontologica Scandinavica 53(3): 129-134.
- Doshi, Patil (2012) A role of genes in craniofacial growth. IIOABJ 3(2): 19-36.
- Alappat S, Zhang ZY, Chen YP (2003) MSX homeobox gene family and craniofacial development. Cell Res 13(6): 429-442.
- Mina M, Gluhak J, Upholt WB, Kollar EJ, Rogers B (1995) Experimental analysis of Msx-1 and Msx-2 gene expression during chick mandibular morphogenesis. Dev Dyn 202(2): 195-214.
- Merlo GR, Zerega B, Paleari L, Trombino S, Mantero S, et al. (2000) Multiple functions of Dlx genes. Int J Dev Biol 44(6): 619-626.
- Zielinski D, Markus B, Sheikh M, Gymrek M, Chu C, et al. (2014) OTX2 duplication is implicated in hemifacial microsomia. PLoS One 9(5).
- Acampora D, Gulisano M, Broccoli V, Simeone A (2001) OTX genes in brain morphogenesis. Prog Neurobiol 64(1): 69-95.
- Blake JA, Ziman MR (2014) Pax genes: Regulators of lineage specification and progenitor cell maintenance. Development 141(4): 737-751.
- Barlow AJ, Bogardi JP, Ladher R, Francis West PH (1999) Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Developmental Dynamics 214(4): 291-302.
- Makarenkova Helen, Meech Robyn (2012) Barx homeobox family in muscle development and regeneration. International Review of Cell And Molecular Biology 297: 117-173.
- Tao W, Chu C, Zhou W, Huang Z, Zhai K, et al. (2020) Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. NatCommun 11(1): 3015.
- Yamada G, Mansouri A, Torres M, Stuart ET, Blum M, et al. (1995) Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 121(9): 2917-2922.
- Choudhry Z, Rikani AA, Choudhry AM (2014) Sonic Hedgehog signalling pathway: a complex network. Ann Neurosci 21(1): 28-31.
- Eberhart, Johann K (2006) Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development 133(6): 1069-1077.
- Xu P, Balczerski B, Ciozda A, Louie K, Oralova V, et al. (2018) Fox proteins are modular competency factors for facial cartilage and tooth specification. Development 145(12): dev165498.
- Graf D, Malik Z, Hayano S, Mishina Y (2016) Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev 27: 129-139.
- Nie XG (2005) Differential expression of Bmp2, Bmp4 and Bmp3 in embryonic development of mouse anterior and posterior palate. Chin Med J 118(20): 1710-1716.
- Lu J, Dai J, Wang X, Zhang M, Zhang P, et al. (2015) Effect of fibroblast growth factor 9 on the osteogenic differentiation of bone marrow stromal stem cells and dental pulp stem cells. Molecular Medicine Reports 11(3): 1661-1668.
- Wen Du, Wei Du, Haiyang Yu (2018) The role of fibroblast growth factors in tooth development and incisor renewal. Stem Cells International 2018.
Article Type
Review Article
Publication history
Received Date: June 20, 2022
Published: July 25, 2022
Address for correspondence
Deepika RS Bais, Department of Orthodontics and Dental Anatomy, Aligarh Muslim University, India
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Sanjeev Kumar V, Aiswareya G, Deepika RS B, Vivek kumar S, Pramod Kumar Y. Gene-to-Face. 2022- 4(4) OAJBS.ID.000473.