Conditions Associated with Type 3 Chlorine Channels (ClC-3)
ABSTRACT
Until a few decades ago, chloride anion channels (Cl-) received little attention in comparison to cationic channels. Nowadays it is known that the chloride ion is the most abundant inside the body and it has been implied in important biological processes, such as: cell volume regulation, transepithelial fluid transport, muscle contraction and neuroexcitation. Furthermore, within the cell, it participates in endosomal, lysosomal and Golgi acidification. Particularly, voltage-gated Cl- channels-3 (ClC-3) mediate extra and intracellular ionic homeostasis and acidification of intracellular compartments. ClC-3 are expressed in most tissues, including brain, retina, pancreas, intestines, epididymis, kidney, liver, skeletal muscle, and heart, so their participation in diseases such as gastric cancer, cervical cancer, endometriosis, osteoporosis and cardiac hypertrophy has been studied.
KEYWORDS
ClC-3; Voltage-gated chloride channels; Chloride channel; ClC-3 diseases
INTRODUCTION
Chloride is the most abundant inorganic ion in the body and, as such, it is considered one of the main osmolytes involved in the regulation of plasma volume, transepithelial fluid transport, muscle contraction and neuroexcitation. Studies show that Cl- flux during plasma volume regulation can occur through ion channels or transporters, resulting in cellular swelling Ernest et al. [1]. Within the cell, chlorine transport across organelle membranes is important in endosomal, lysosomal, and Golgi acidification. Chlorine channels are the best route for transmembrane transport in these processes Verkman [2]. Chlorine channels are ubiquitous and are located both in the plasma membrane and in the membrane of intracellular organelles. They have different cellular functions, among which we can mention the regulation of electrical excitability, ionic homeostasis, regulation of pH levels and cell volume, the latter being particularly important for the migration of cancer cells. These channels are also involved in cell cycle regulation, probably as key factors in the progression from G1 to S phase Peretti et al. [3].
Chlorine channels show low selectivity for other inorganic anions (Br-, I-, Cl-), and some allow the passage of organic anions, for which reason it has been proposed to refer to them in a general way as “anion channels”; however, the term “chlorine channel” may be more appropriate since chloride is the predominant anion in biological systems Duran et al. [4].
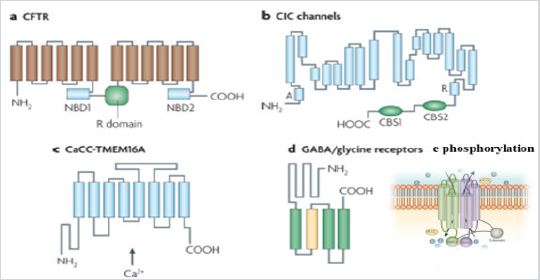
According to their opening mechanisms, chlorine channels are classified into five subtypes: cystic fibrosis transmembrane conductance regulators (CFTR, for its acronym in English Cystic fibrosis transmembrane conductance regulator), which is activated by dependent phosphorylation. of cAMP; calcium-activated chlorine channels (CaCC); voltage-gated chlorine channels (ClC); ligand-dependent chlorine channels and volume-regulated chlorine channels (Figure 1).
The discovery of ClC proteins in the early 1990s was important for the development of Cl- transport research. ClC proteins form a large family that regulates the voltage-dependent transport of Cl- ions across the membrane. They are expressed both in plasma membranes and in intracellular membranes of the cells of almost all organisms. There are four mammalian ClC proteins (ClC-1, -2, -Ka, and -Kb) that act as voltage-gated anion channels in the plasma membrane. ClC channels generally show a sequence selectivity towards Cl-> Br-> I-, have low cationic permeability and are homodimeric. ClC-3, -4, -5, -6, and -7 proteins regulate Cl-: H + exchange rather than voltage-dependent anion channel activity. Unlike channels, exchange subtypes are generally found on intracellular membranes. They have a complex topology with 18 segments, eight of which form hairpin structures that do not completely cross the membrane, this arrangement makes it possible for residues from distant parts of the protein to be together in the center of the subunit, forming the selective filter (Figure 1). The carboxyl terminal of ClCs in eukaryotes contains two cystathionine- β-synthase motifs important in channel modulation. One of the transmembrane segments contains a highly conserved glutamate residue that is crucial for both channel and Cl-transport functions. In channel functions, the movement of carboxyl glutamate in and out of the pore appears to have implications on the speed of channel opening Verkman [2]; Poroca et al. [5]; Duran et al. [4].
Mutations in the genes that encode ClCs proteins are associated with genetic disorders such as myotonia and osteopetrosis, the first is a muscle disorder characterized by a delayed relaxation of skeletal muscle after voluntary contraction, and the second is a A rare disorder that causes bones to grow abnormally and become too dense Verkman [2].
Channels ClC-3
Chlorine channel-3 (ClC-3), encoded by the CLCN3 gene, is a member of the family of voltage-gated chlorine channels that primarily mediates extra and intracellular ionic homeostasis and acidification of intracellular compartments. ClC-3 is expressed in most tissues, including brain, retina, pancreas, intestines, epididymis, kidney, liver, skeletal muscle, and heart. Recent studies have shown that ClC-3 participates in the processes of proliferation, migration, invasion and apoptosis Guan et al. [6]; Poroca et al. [5].
Prostate Cancer and ClC-3
ClC-3 channels are expressed in lymph node carcinoma of epithelial cells of prostate cancer (LNCaP). Inhibition of ClC-3 by specific antibodies effectively prevents turgor-activated chlorine currents in LNCaP cells. This suggests that ClC-3 may be the turgoractivated chlorine channel in these cells. Furthermore, BCl2 (B-cell lymphoma 2) increases the expression of ClC-3, as well as the swelling-activated chlorine currents, indicating that ClC-3 mediates the BCl2-dependent modulation of the swelling-activated chlorine currents. Since cell proliferation is associated with cell volume changes, ClC-3 appears to contribute to volume changes during LNCaP cell proliferation Lemonnier et al. [7].
In 2008, Mao et al. Demonstrated that ClC-3 is overexpressed in nasopharyngeal carcinoma cells and can regulate cell migration, which is one of the events in tumor metastasis and malignant transformation, frequently accompanied by improved tumor cell mobility, which plays an important role in the control of cell proliferation, making it a target for cancer therapies Mao et al. [8].
Gliomas and ClC-3
Gliomas are one of the most common brain tumors in adult humans. It has been shown that ClC-3 is found at high levels in glioma membranes, in addition to participating in an important way in cell migration and in the secretion of chlorine ions, since it has been suggested that this channel is the main contributor in the exit of these ions Lui et al. [9]. In 2013 investigated the expression and function of ClCs in malignant glioma cells and found that ClC- 3 is the chlorine channel associated primarily with invasiveness; however, just blocking it was not enough to completely inhibit glioma cell invasion Lui et al. [9].
Menopause and CCl3
In 2016, Guan et al. [10], conducted a study with forty-three premenopausal women between 27 and 45 years of age with endometriosis in different stages (I, II, III and IV), in which they observed that the levels of ClC-3 were significantly elevated , in addition to being distributed throughout the cell, especially in the cytoplasm, which suggests that ClC-3 may be associated with the development of this disorder by facilitating the migration and invasion of endometrial stromal cells, therefore high levels ClC-3 could be a potential prognostic for the development of endometriosis since they are also related to clinical symptoms such as chronic pelvic pain, dysmenorrhea, dyspareunia and infertility Guan et al. [10].
Gastric Cancer and CCl3
A study in 2018 showed that ClC-3 was overexpressed in gastric cancer tissues and that this overexpression is a poor prognostic biomarker: however, the high expression of ClC-3 was correlated with a deeper tumor invasion, an increase in lymph node metastasis and more advanced clinical status. Cell proliferation and migration were shown to be the primary biological functions of ClC-3 in gastric cancer cells Guan et al. [10].
Osteoporosis and CCl3
Morbidity due to osteoporosis presents specific differences depending on gender. Estrogen deficiencies during menopause lead to osteoporosis, which is a systemic metabolic disease that typically occurs after menopause and is accompanied by a reduction in bone density and an increase in fragility and risk of fracture. In 2018, Deng’s group studied the role of chlorine channels in the regulation of estrogen in osteoblasts. Osteoblasts are responsible for bone formation and are controlled by estrogen levels during bone remodeling. They found that estradiol promotes the expression of ClC-3 channels, and that they regulate osteodifferentiation in osteoblasts; in such a way that, by silencing the expression of ClC-3, the elevation of osteodifferentiation in estrogen-regulated osteoblasts is prevented. These data suggest that estrogen regulates bone formation by activating ClC-3 chlorine channels and that, in turn, ClC-3 channel activation can enhance ostedifferentiation in osteoblasts Deng et al. [11].
Cardiac Hypertrophy and CCl3
Cardiac hypertrophy is characterized by an increase in the size of the cardiomyocytes, an excess of protein synthesis, and a thinning of the ventricular walls. In 2018, Li et al. Reported that ClC- 3 expression was reduced in hypertrophic H9c2 cells (embryonic rat cardiomyocytes), neonatal rat cardiomyocytes and mouse myocardium, and this reduction in ClC-3 expression could be prevented in pretreated mice. with propanolol, demonstrating that ClC-3 plays an important role in β-adrenergic cardiac hypertrophy which can be attributed to the reduction of type A natriuretic peptide (ANF) and β-myosin heavy chain (β-MHC), so that ClC -3 may be a novel therapeutic target for the prevention or treatment of myocardial hypertrophy Li et al. [12].
Cervix and CCl3
Recently, Guan et al. Investigated the relationship between ClC- 3 expression levels and its clinical importance in human cervical carcinoma to illustrate its impact on the progression of cervical cancer, one of the most important gynecological cancers causing thousands of cancer-related deaths in women around the world. In this study, they found that the expression levels of mRNA and ClC-3 protein were increased by 60% in paracancerous tissues and cervical carcinomas. The ClC-3 protein was mainly expressed in cervical epithelial cell cytoplasm, indicating that ClC-3 is strongly associated with cervical epithelial cell homeostasis and malignancy. They also described that the expression of ClC-3 is associated with the pathological characteristics of the patients, which suggests that ClC-3 may be a marker for cervical carcinoma [13].
CONCLUSION
Within the chlorine channels we find the family of proteins called ClC, particularly it has been observed that ClC-3 plays important roles in specific cellular functions, it has been possible to study its expression in different types of diseases, among which we can mention cervical cancer, endometriosis, osteoporosis, cardiac hypertrophy, gastric cancer, nasopharyngeal carcinoma, gliomas, and prostate cancer. These studies propose that ClC-3 can be useful as a marker of diseases and even a therapeutic target for their prevention or treatment.
REFERENCES
- Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW (2005) Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol 288(6): C1451- 1460.
- Verkman AS, Galietta LJ (2009) Chloride channels as drug targets. Nat Rev Drug Discov 8(2): 153-171.
- Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, et al. (2015) Chloride channels in cancer: Focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta 1848(10 Pt B): 2523-2531.
- Duran C, Thompson CH, Xiao Q, Hartzell HC (2010) Chloride channels: often enigmatic, rarely predictable. Annu Rev Physiol 72: 95-121.
- Poroca DR, Pelis RM, Chappe VM (2017) ClC channels and transporters: structure, physiological functions, and implications in human chloride channelopathies. Front Pharmacol 8: 151.
- Guan YT, Huang YQ, Wu JB, Deng ZQ, Wang Y, et al. (2016) Overexpression of chloride channel-3 is associated with the increased migration and invasion ability of ectopic endometrial cells from patients with endometriosis. Hum Reprod 31(5): 986-988.
- Lemonnier L, Shuba Y, Crepin A, Roudbaraki M, Slomianny C, et al. (2004) Bcl-2-dependent modulation of swelling-activated Cl- current and ClC-3 expression in human prostate cancer epithelial cells. Cancer Res 64(14): 4841-4848.
- Mao J, Chen L, Xu B, Wang L, Li H, et al. (2008) Suppression of ClC-3 channel expression reduces migration of nasopharyngeal carcinoma cells. Biochem Pharmacol 75(9): 1706-1716.
- Lui VC, Lung SS, Pu JK, Hung KN, Leung GK (2010) Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res 30(11): 4515-4524.
- Guan YT, Xie Y, Zhou H, Shi HY, Zhu YY, et al. (2019) Overexpression of chloride channel-3 (ClC-3) is associated with human cervical carcinoma development and prognosis. Cancer Cell Int 19: 8.
- Deng Z, Li W, Xu J, Yu M, Li D, et al. (2018) ClC-3 chloride channels are involved in estradiol regulation of bone formation by MC3T3-E1 osteoblasts. J Cell Biochem.
- Li C, Huang D, Tang J, Chen M, Lu Q, et al. (2018) ClC-3 chloride channel is involved in isoprenaline-induced cardiac hypertrophy. Gene 642: 335- 342.
- Gu Z, Li Y, Yang X (2018) Overexpression of CLC-3 is regulated by XRCC5 and is a poor prognostic biomarker for gastric cancer. J Hematol Oncol 11(1): 115.
Article Type
Review Article
Publication history
Received Date: December 23, 2021
Published: January 19, 2022
Address for correspondence
Claudia Mancilla-Simbro, Laboratorio de Biofísica Cardiaca, Instituto de Fisiología. Benemérita Universidad Autónoma de Puebla, México
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Claudia MS, Erwin JPC, José EHM, Sandra OG, Gerardo GAE, José AGD, Dajiehdy PM, Citalli GE, Alberto RM. Conditions Associated with Type 3 Chlorine Channels (ClC-3). 2022- 4(1) OAJBS.ID.000379.