An Elusive Diagnosis - Posterior Cortical Atrophy
ABSTRACT
Posterior Cortical Atrophy (PCA) is a neurodegenerative syndrome characterized by progressive apraxia, dyslexia and most strikingly, loss of visual perceptual and visuospatial ability. In most cases, neuro-degeneration of the occipital, parietal, and posterior temporal cortices occurs as a consequence of the Visual Variant of Alzheimer disease (AD). Less frequent etiologies include Corticobasal Degeneration, Dementia with Lewy bodies (DLB), and the Heidenhain variant of Prion disease. It is unknown what predisposes these conditions to present as PCA. Given its rarity and variable presentations, misdiagnosis is common. We present a case of a 60-year-old woman with PCA who was initially undiagnosed. She measurably improved on donepezil and memantine suggesting AD as its pathogenesis.
INTRODUCTION
PCA is a neurodegenerative syndrome typified by a progressive deterioration in visual processing abilities. It can also affect other processes that localize to the occipital, parietal, and posterior temporal regions. PCA typically manifests during the 5th and 6th decades of life. It remains understudied when compared to other neurodegenerative processes [1]. We believe that diagnosis is often significantly delayed due to its variable presentation and obscure symptoms.
Balint syndrome was first described in 1909 by Rezes (Rudolf) Balint [2]. It comprises the triad of optic ataxia (inability to accurately reach for objects), oculomotor apraxia (inability to generate voluntary saccades to track objects in the visual field), and simultanagnosia (inability to perceive a complex visual scene composed of multiple objects).
The mechanism may be impaired short-term visual memory, referred to as a “visual binding deficit” by Parra and Abrahams in 2010 [3]. The syndrome is a consequence of damage to bilateral parieto-occipital cortices. It may be seen as a late manifestation of Alzheimer disease.
It is also observed in cases of trauma, stroke, and intracranial neoplasm. Rarely, it can be seen in frontal lobe injury, likely secondary to a disconnection between the frontal eye fields (generator of saccades) and parietal lobe (locating an object in space).
CASE REPORT
A 60-year-old female presented to our clinic after 3 months of increasing difficulty “using her eyes.” Cataract extraction did not alleviate the symptoms. For 3 years, she had difficulty writing; letters were upside down or illegible. She had trouble reading and could not recite her phone number. She approached the wrong side of the car when entering. When prompted to look left or right, she was unable to do so. She had poor hand-eye coordination when reaching for an object. When asked to cover an eye, she placed her hand next to her forehead covering only empty space. She found it difficult to dress properly (dressing apraxia). She denied headache, head trauma, stroke, or cancer. Her mother suffered from an unspecified dementia.
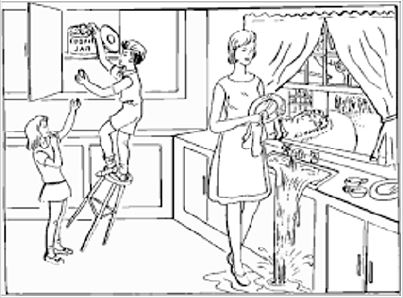
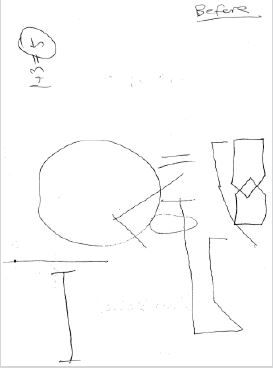
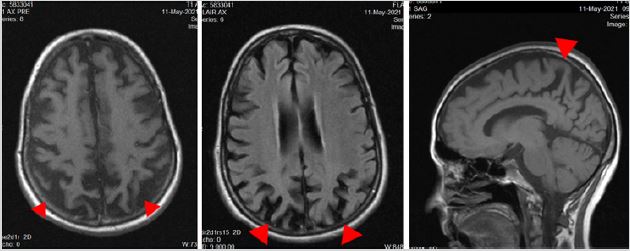
On examination, she was able to count fingers in all visual fields. Her pupils reacted normally. She had no dysarthria. She could name simple items and repeat phrases without error. She had difficulty interpreting the Boston Cookie Theft Scene (Figure 1). She described single items but could not appreciate their meaning within the scene (i.e. simultagnosia). She identified water on the floor and had to trace it up to the sink before realizing it had spilled. She spotted a child but could not tell he was falling off a stool. She was unable to bisect a line, drawing her line apart. She was unable to copy overlapping pentagons. She failed to place the hands on a blank clock face to show 11:10. She wrote the numbers eleven and ten apart from the clock and misplaced the hands. She incorrectly calculated the sum of 3 plus 1 as 5 (Figure 2). She had difficulty identifying relatives in a group photograph. She had trouble mimicking 3 step hand sequences (palm down, palm up, karate chop thumb up) with either hand. With eyes closed, she had difficulty identifying letters traced on her left palm. She had trouble naming her fingers, e.g., thumb. Her MRI brain revealed bilateral posterior cortical atrophy (Figure 3).
After five months of treatment with donepezil and memantine she and her husband reported modest improvement in her brain fog. On exam, she had improved in some skills.
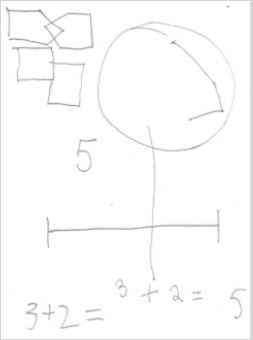
She was unable to draw a clock to say 2:15. She was unable to copy overlapping pentagons. She drew a line off center when asked to bisect it. She correctly added 3 plus 2 (Figure 4).
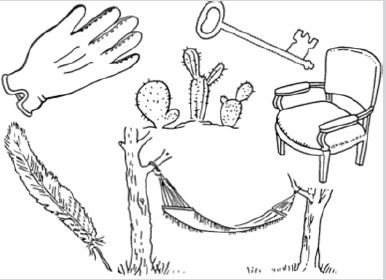
Her description of the Boston Cookie Theft Scene (Figure 1) remained impaired, isolating single elements out of context. After a noticeable delay, she recognized that a child was falling off a stool and water was overflowing the sink. She was able to label her fingers correctly. She walked the room with confidence. On the Boston Naming Test (Figure 5). She could not identify a hammock attached to trees, but identified the trees. She was able to recognize the face of Abraham Lincoln. She could name a horse-shoe charm on her bracelet. She misidentified a pen as a pencil but recognized its color. She was able to grab the right thumb positioned in front of her with eyes closed. She was able to perform finger to nose without dysmetria. Her eyes had saccadic undershoot from far right to the center but not from the far left.
DISCUSSION
The core findings of PCA include insidious onset with gradual progression, relatively preserved episodic memory, visual deficits not caused by eye pathology, verbal fluency, insight, the presence of Balint syndrome (i.e. optic ataxia, ocular apraxia, optical agnosia, simultanagnosia, and environmental disorientation), and absence of intracranial tumors or stroke [1]. Supportive features include onset before 65 years old, ideomotor apraxia (difficulty demonstrating a task e.g. how to hammer a nail) alexia (difficulty reading), acalculia (inability to perform simple arithmetic), agraphia (inability or regression in writing), and posterior cortical atrophy or hypoperfusion on neuroimaging.
Several factors influence the prognosis of PCA. These include the interval between symptom onset and presentation, the type of deficit, underlying cerebral pathology, and the presence of anxiety or depression [4]. Common complaints include difficulty reading text, judging distances (resulting in repeated car accidents on the road or while parking), locating and recognizing objects by sight, and trouble on stairways or escalators [5]. Optical distortions may be misinterpreted as migraine symptoms.
PCA can present in patients as young as 50 years old. The average time between symptom onset to diagnosis is four years. An estimated 65% of PCA patients visit an ophthalmologist prior to diagnosis [6]. In those diagnosed with PCA, 88% had complete or partial Balint syndrome, 62% complete or partial Gerstmann syndrome (left-right confusion, finger agnosia, agraphia, acalculia), and 48% had visual field loss [7].
Out of 523 Alzheimer patients seen at a memory disorder clinic 24 (5%) had visual difficulties and 13 (3%) had apraxia upon initial presentation [8]. Another study reported that in patients with AD, those with PCA tend to develop visual deficits with or without apraxia prior to the onset of short-term memory loss [9].
PCA symptoms start by the mid-50s to early 60s. Women are over-represented in this studies [7]. The prevalence of PCA is unknown. The rate of disease progression depends on the underlying neuropathology. In those with the Visual Variant of AD, life expectancy typically exceeds 5 years. With the Heidenhain Variant of Prion disease, duration is reported up to two years, which is longer than months seen in wild type Prion disease [10].
At present, there are no randomized, double-blind, placebocontrolled trials assessing the efficacy of acetylcholinesterase inhibitors (e.g. rivastigmine, galantamine, donepezil) in PCA. They are often prescribed since AD is the most common pathobiology. There use was reported beneficial, likely in those with underlying DLB or AD. Patients with persistently depressed mood may benefit from antidepressant medication.
Those with parkinsonism may benefit from levodopa/carbidopa. Physical therapy may help maximize patient functionality. Caregiver Support groups are beneficial for relieving social isolation and for providing a forum to share experiences and coping tactics [6].
Our patient was undiagnosed for years and continued to experience visual difficulties in spite of cataract removal. It was only then that she sought the input of a neurologist. Treatment with donepezil and memantine had a positive effect on her symptoms. Providing a diagnosis to the patient and her husband removed uncertainty and began the process of preparing for the future.
CONCLUSION
PCA is a neurodegenerative syndrome whose diagnosis is often delayed or missed due to lack of familiarity with its presentation. In our case, pharmaceuticals used for AD improved symptoms.
REFERENCES
- Schott JM, Crutch S J (2019) Posterior cortical atrophy. Continuum 25(1): 52-75.
- Parvathaneni A, Das J (2021) Balint Syndrome. StatPearls Publishing, USA.
- Brown LA, Niven EH, Logie RH, Rhodes S, Allen RJ (2017) Visual feature binding in younger and older adults: encoding and suffix interference effects. Memory 25(2): 261-275.
- Glazer H, Saadatpour L, Doty L, Heilman KM (2017) A case of posterior cortical atrophy with vertical neglect. Neurocase 23(2): 114-119.
- Putcha D, McGinnis SM, Brickhouse M, Wong B, Sherman JC, at al. (2018) Executive dysfunction contributes to verbal encoding and retrieval deficits in posterior cortical atrophy. Cortex, 106, 36-46.
- Mendez MF, Khattab YI, Yerstein O (2021) Impaired visual search in posterior cortical atrophy vs. typical Alzheimer’s disease. J Neurol Sci 428: 117574.
- Picillo M, Scannapieco S, Iavarone A, Ginevrino M, Valente EM, et al. (2021) Posterior Cortical Atrophy phenotype in a GBA N370S mutation carrier: a case report. BMC neurology, 21(1): 1-4.
- Migliaccio R, Agosta F, Basaia S, Cividini C, Habert MO, et al. (2020) Functional brain connectome in posterior cortical atrophy. NeuroImage Clin 25: 102100.
- Baiardi S, Capellari S, Ladogana A, Strumia S, Santangelo M, et al. (2016) Revisiting the Heidenhain Variant of Creutzfeldt-Jakob Disease: Evidence for prion type variability influencing clinical course and laboratory findings. J Alzheimer’s dis 50(2): 465-476.
- Montembeault M, Brambati SM, Lamari F, Michon A, Samri, D, et al. (2018) Atrophy, metabolism and cognition in the posterior cortical atrophy spectrum based on Alzheimer’s disease cerebrospinal fluid biomarkers. NeuroImage Clin 20: 1018-1025.
Article Type
Case Report
Publication history
Received Date: January 18, 2022
Published: January 28, 2022
Address for correspondence
Asia Filatov MD, Florida Atlantic University, Charles E. Schmidt College of Medicine, Boca Raton, USA
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Asia F, Jonathan TG, Marc AS. An Elusive Diagnosis - Posterior Cortical Atrophy. 2022- 4(1) OAJBS.ID.000386.
Figure 1: Boston cookie theft scene.
Figure 2: Patient form on initial presentation April 2021 diagnosed with PCA.
Figure 3: MR Head (right to left: T1 Axial, FLAIR Axial, T1 Sagittal). Red arrow heads illustrate posterior atrophy.
Figure 4: 5 months after treatment with donepezil and memantine.
Figure 5: Boston naming test.