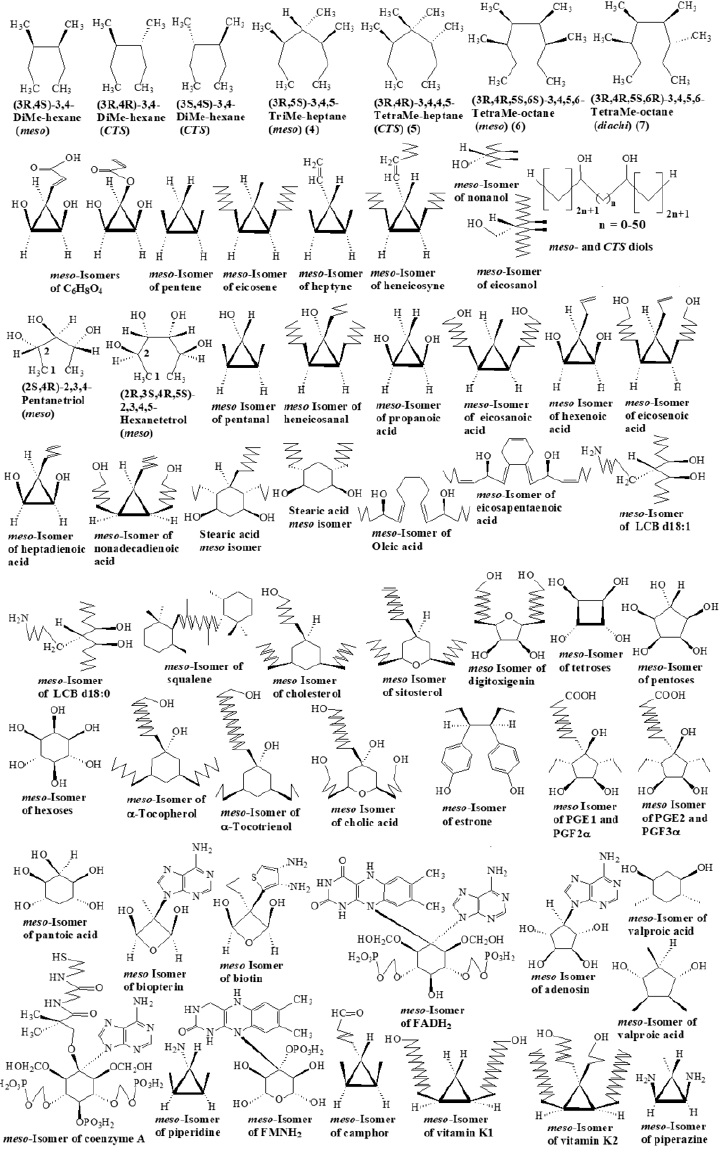
All the Major Metabolites Containing a Significant Aliphatic Moiety Possess At Least One Real or Envisaged Meso Isomer
ABSTRACT
The premise of this paper is that every molecular formula of a compound containing a significant moiety of aliphatic skeleton, and above a given degree of complexity, presents four types of isomers:(a) meso; (b) C2 symmetrical (CTS); (c) diastereomeric chiral (diachi); (d) diastereomeric chiral (diachi). The definitions of the four types are improved and updated according to nowadays data. Moreover, meso isomers have been selected as structural reference. In molecules with a minimum unsaturation, the skeleton of the first four cycloalkanes have been used in order to present the structure of the envisaged meso isomers. In fact, only cyclobutane, cyclopentane and cyclohexane derivatives are able to present the first three types of isomers. A very wide basis of compounds, and of a variety of classes, has been approached. We have paid the due attention to plausible, envisaged isomers as an instrument of interpretation.
KEYWORDS
Isomers; Meso; C2 symmetrical (CTS); Diastereomeric chiral (diachi); Constitutional; comparative chemistry
INTRODUCTION
Different classes of compounds develop at varying pace, and when they reach a certain extent, they should become independent. According to a previous definition, diastereomers are all isomers that are not enantiomers [1,2] Since this group became too heterogenous we have fragmented it in four parts: meso, CTS and constit.; in the diachi group, we have kept only chiral members. It’s obvious that all these groups [2-6], except meso [7] appreciably increased, hence they deserve an independent place. For this reason, an updating of terms and systematization is periodically needed [8,9]. We have undertaken an integrative approach based on molecular formula, and an exercise of comparative chemistry, by using meso compounds as structural references. Every molecular formula concerning natural or synthetic compounds, with a significant moiety of an aliphatic skeleton, and a given level of complexity, might produce the above mentioned four types (groups) of isomers. In fact, the significance given by us to the four types will become quite obvious from their use and application.
There are three types of meso compounds: (A1) the molecule of the first type is formed of two enantiomeric chiral halves uniformly linked with each other [10-12] (A2) the two enantiomeric chiral halves of the second type are uniformly linked on an atom or on a matrix devoid of handedness or characterized by a mirror plane of symmetry [13] (A3) The third type of meso compounds is devoid of elements of symmetry (dissymmetric compounds) and they have to be analyzed by Cahn-Ingold-Prelog rules [14,15]. Their molecule contains two sets of asymmetric carbons with opposed handedness. (In the latter case one can assert that molecules are formed of two imaginary enantiomeric halves separated by an imaginary mirror plane of symmetry). (A1) and (A2) are characterized by a mirror plane of symmetry. Meso are optically inactive (optinactive) due to an internal compensation. The existence of two enantiomeric sides in meso compounds was proved by [16] in an elegant experiment on galactaric acid. They kinetically reduced this acid, and the product was a racemic mixture of galacturonic acids. The two enantiomeric acids were separated as strychnine salts and characterized. In this way the internal enantiomorphy of meso derivatives become externalized. Subsequently, chemists would try to overturn this feature of meso compounds and to predominantly, if not exclusively, prepare one product only [17-19].
Meso heterodimers polyols discovered (invented) by Fischerxylitol [20], ribitol (adonitol) [21], xylaric acid, ribaric acid ‒ have been a trailblazing achievement and they turned out to be models for other combinations of the same category. Mirror plane of symmetry has to be regarded as an intrinsic property of meso compounds. It should be considered both a physical instrument and a natural phenomenon. Mirror plane of symmetry cuts either a bond (bonds) or atoms. Relative to polarized light, mirror plane of symmetry transforms a heterodimer into a homodimer. Mirror plane of symmetry hides (masks) the atoms cut by it from polarized light, and what remains, as evidenced by this physical instrument, is an entity containing an even number of atoms, i.e. a homodimer. Meso heterodimers constitute a chemical duality, the two opposed sides of duality are their heterodimeric character, on one hand, and their expression as homodimers. According to Kelvin and Prelog theory [22-25] meso compounds are internally heterochiral. C2 symmetrical (CTS) compounds have been defined in relation with an axis and a rotation of 180°. After this maneuver the same atoms should be regained as initially [26-28], and all CTS compounds are chiral and optically active (optactive). Their molecule is either formed of two identical chiral halves uniformly linked with each other [29,30] or of two identical chiral halves uniformly linked on an atom or on an achiral or CTS matrix [31]. According to Kelvin and Prelog theory CTS formed exclusively of two identical chiral halves are homochiral with each other and internally homochiral [32]. Of this reason, they could be named also twin molecules [33]. The exceptional properties of twin (CTS) compounds were also noticed by [34]. Homodimeric CTS compounds constitute a chemical duality, the two opposed sides of duality are optical activity, on one hand, and their symmetry. There is one universal rule concerning CTS compounds: every member of this group possesses a real or imaginary meso isomer, but the reverse is not necessarily true: some meso isomers are devoid of a matching CTS representative. Two cases should be mentioned. Compounds based on 1,2-diamino-cyclohexane [35] are CTS as long as they are trans. However, they are not necessarily meso when they are 1,2-cis. (Cis-isomer is written in this case as 1,2-cyclobutane derivative). On the other hand, of the six meso isomers of inositol five are characterized by 1,4 mirror planes of symmetry, while allo-inositol is devoid of such a plane. Its meso nature ca be explained only by a planar structure, hence the mirror plane of symmetry cuts two opposed bonds. The first CTS combinations, the two enantiomers of tartaric acid, have been separated by [36] by crystalization from a racemic mixture that had been prepared by Kestner [37,38] Pasteur noticed two types of crystals, that were enantiomorphic with one another. He separated the two types of crystals and found out that their aqueous solutions were dextrorotary and levorotary, respectively. Dextro-tartaric acid had been discovered by Scheele 1770 in the sediment deposited in the vats during the grape juice fermentation [39,40]. Another isomer, devoid of optical activity and not cleavable by any chemical or biological method, was discovered also by Pasteur 1853 and called meso-tartaric acid [41] Stereochemical theory of tetrahedral and asymmetric (chiral) carbon atom [42,43] led van’t Hoff to molecular models based on tetrahedrons which unequivocally represented every chiral carbon atom. By constructing and using these models, van’t Hoff expanded the idea of enantiomorphism from crystals to molecules. (Dots and wedges representations of today come from Van’t Hoff’s models). However, at that time no scientist could rationally associate structural models with the two enantiomers [44]. In fact, the discovery of Pasteur increased the dilemma of representation, i. e., the relationship between a sample of an optically active compound and the unique, characteristic, structural model possibly assigned to it. This dilemma was solved by X-ray diffraction, i. e., of zirconium Kα rays, by sodium rubidium tartrate of the dextrorotary species, and the obtained model was assigned to (+)-tartaric acid [45]. By an impressive coincidence, this configuration of (+)-tartaric acid had been hypothetically attributed by [46]. Configuration of chiral centers of (–)-tartaric acid became also known, by the virtue of the law of enantiomorphism. Configuration of the two enantiomers has been connected with other chiral compounds, beginning with (‒)- and (+)-glyceraldehyde [47]. A chemical relationship has been found between [48], due to a derivative of D- and L-mannitol prepared by the latter, i.e. 1,2-5,6-di-O-isopropylidene mannitol (CTS). By integration of finding of [49] in the strategy of E. Fischer, structure elucidation of linear aldohexoses becomes more direct [50].
The third group is formed of chiral diastereomers which possess a carbon skeleton identical to meso and CTS, i.e. a phenomenon of isoskeletomeric relationship [51]: bicubebin [52], bismurrangain [53], hybocarpone quadrigemine C [54], numerous carotenoids [55]. However, their asymmetric carbons are distributed in an irregular manner in comparison with meso or CTS. We have called them diachi (from diastereomeric chiral). Meso isomers are characterized by a 1:1 ratio of numbers of R and S carbons while in CTS ones this ratio is n:0, 0:n or 1:1. In diachi combinations the ratio R/S has other values.
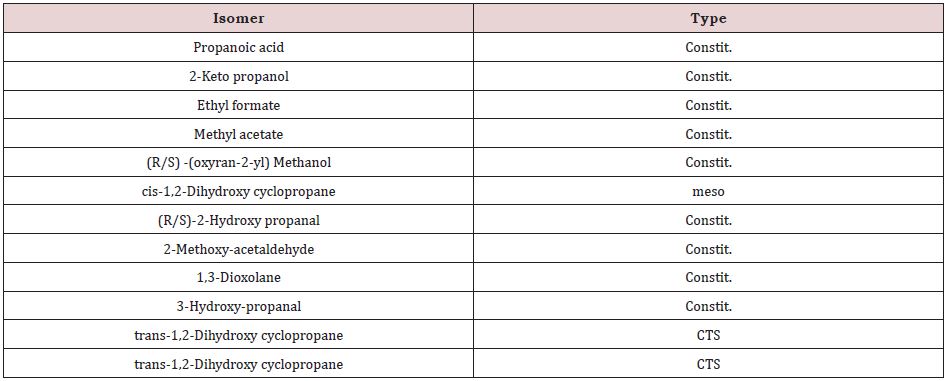
Constitutional (positional) isomers form the fourth group. They are isomer with the preceding ones but their skeleton is different. They are either optactive or optinactive. With relatively few exceptions, compounds currently met in living things are constitutional isomers. Constitutional isomers present probably the highest molecular diversity. At least two dozen of isomers with molecular formula C3H7NO2 can be written, without any expedient or phantasy, just by utilising the concecrated valence of every component element. However, of the envisaged isomers only some present elements of symmetry: two are meso (cis-1,2-dihydroxy-3- amino cyclopropane and cis-2,4-dihydroxy-azetidine) and two are CTS (trans-2,4-dihydroxy-azetidine, two enantiomers), and all the other, including (R)- and (S)-alanine, are constit.
An interesting group of constit. isomers are formed by a nonuniform likage of monomers: aspergilazine A [56], penicillixanthone A, phomoxanthone B, dideacetylphomoxanthone B, rugulotrosin B [57] quadrigemine B [54], taondiol dimer [58,59], numerous carotenoids.
The application of our systematization to monosaccharides discovered/invented by Fischer and others produces the following results.
a) Meso monosaccharides: galactitol [(2S,3R,4S,5R)- hexitol] [60]; allitol [(2S,3S,4R,5R)-hexitol] [61-64], galactooctitol [(2S,3S,4R,5S,6R,7R) octitol]; [65-67], galactaric acid (2R,3S,4R,5S) allaric acid (2R,3R,4S,5S) [68]. (B) CTS monosaccharides: D-mannitol [(2R,3R,4R,5R)-hexitol], L-mannitol [(2S,3S,4S,5S)- hexitol] D-mannaric acid [(2S,3S,4S,5S)], L-mannaric acid [(2R,3R,4R,5R)]; [69-73), D-iditol [(2R,3S,4S,5R)-hexitol], L-iditol [(2S,3R,4R,5S)-hexitol], D-idaric acid [(2R,3S,4S,5R)], L-idaric acid [(2S,3R,4R,5S)]; [74-76].
b) Diachi monosaccharides: D-glucitol [L-gulitol (2S,3R,4R,5R)-hexitol] [77], L-glucitol [D-gulitol (2R,3S,4S,5S)- hexitol], D-glucaric acid [L-gularic (2R,3S,4S,5S)], L-glucaric acid [D-gularic (2S,3R,4R,5R)], D-altitol [D-talitol (2R,3S,4R,5R)- hexitol], L-altitol [L-talitol (2S,3R,4S,5S)-hexitol], D-altraric acid [D-talaric (2S,3R,4S,5S)], L-altraric acid [L-talaric (2R,3S,4R,5R)] [78-80] and 1,1,1,2,2,3-hexanehexol [81].
c) Constitutional: D-hamamelitol [82-84]. Concerning limits and possibilities of reciprocal changing of types mentioned above, both CTS and irrechi can be transformed into meso but the reverse is not true: there are meso compounds that cannot be turned to CTS and irrechi. Eg. trihydroxy alkanes, trimethyl alkanes, i.e. vicinal trisubstituted alkanes. Some interesting facts: the molecule of iditols and idaric acids possesses an equal number of R and S carbons, similarly with galactitol, allitol, galactaric and allaric acids. However, they are not meso but optactive [85]. The difference can be explained probably by the fact that the molecule of the former is formed of two identical chiral halves and the latter of two chiral enantiomeric halves. The two hydrogen atoms of central methylene of a meso derivative, i.e. 3-deoxyxylitol, 3-deoxyribitol, meso-diaminopimelic acid, etc., are not equivalent. (If they are alternatively replaced by a hydroxy function, the products are different). The two central hydrogen atoms of CTS compounds, i.e. 3-deoxyarabinitol, 3-deoxylyxitol, L,L- and D,D-diaminopimelic acid, etc., are equivalent: if they are alternatively replaced by a hydroxy function, exclusively one product is obtained.
The molecular diversity is connected with the following factors:
a) Structures as diamond [86], graphite and fullerenes [87,88] illustrate the best the ability of C atoms to bind with each other. However, all these forms present a very limited structural variety.
b) What really confer molecular diversity to C combinations is the association of this element with hydrogen and this is evidenced by the remarkable molecular variety of aliphatic hydrocarbons [89]. Molecular diversity is a physical-chemical magnitude concerning the ability of a compound to present a large number of isomers.
c) Chemical functional groups, in relative low proportion, also favor molecular diversity. Aromatic hydrocarbons presents the lowest molecular diversity of all organic combinations. They contain an exceeding number of chemical functions, and they are in a state of advanced oxidation. In fact, they fill an intermediate place between elementary carbon and aliphatic hydrocarbons. Another remarkable feature of aromatic hydrocarbons is the fact that they do not present meso isomers.
d) Molecular diversity increases exponentially with molecular weight Roberts [90]; Fujita [32].
e) Carbon dioxide is a terminal facet of metabolism and combustion of organic compounds. It is characterized by a high chemical inertia. Carbon dioxide has to be attached to a preexisting structure, as a piece of metal in a lathe, and stepwise reduced, the energy of sun playing an essential role in this process. Our aim were especially monomeric units [91], but we prove that compounds called by Metzler in this way can also have an authentic dimeric character.
THE MAJOR METABOLITES CONTAINING A SIGNIFICANT ALIPHATIC MOIETY POSSESS AT LEAST ONE REAL OR ENVISAGED MESO ISOMER
Compounds containing cyclopropane or cyclobutane ring, either as cycloalkanes or as heterocycles are more and more numerous [92- 94]. Cis- and trans-1,2-dimethyl cyclopropane is indistinguishable of thermodynamic point of view [95]. 1,2,3-Trihydroxycyclopropane is known as an unstable combination, however no attempt was made to stabilize it [96,97]. 1,2-Dihydroxycyclopropane has been prepared by a reduction reaction of a diketone derivatives [98]. Cis-1,2-dihydroxycyclopropane has been discovered in natural material as a glycoside of α-D-galactopyranose [99]. Two syntheses of cis-1,2,3,4-tetrahydroxy cyclobutane have been reported [100].
A double bond has been usually represented by a ring (cycle). For the construction of isomers of such compounds we have extensively used cycloalkanes as cyclopropane, cyclobutane, cyclopentane, cyclohexane, as well as heterocycles: azetidine, oxetanes, thietanes, tetrahydrofuran, tetrahydropyran [101,102]. Other principles used by us will be obvious from the presented structures. We have made an attempt to present all isomers connected with the molecular formula C3H6O2; (Table 1). It is obvious that constit., isomers are prevailing. There are two CTS isomers and one meso, while diachi is missing.
Construction of a meso isomer for molecular formula C4H4O4 (maleic or fumaric acid) fails, since unsaturation per mol is identical to benzene. However if one add two methylenes (2,3-dimethyl maleic acid, etc., C6H8O4), meso representation is possible (Figure 1).
Hence, we asserted that meso compounds would be a structural reference, and for every set of compounds with the same molecular formula we’d try to find one (or more) real or envisaged meso isomers. For alkanes this degree of complexity begins with C8 (Figure 1): (3R,4S)-3,4-DiMe-hexane (meso), (3R,4R)-3,4-DiMehexane (CTS), (3S,4S)-3,4-DiMe-hexane (CTS), n-octane (constit), etc.; C8 doesn’t present diachi. C9 presents the same type of isomers as C8: (3R,5S)-3,5-diMe-heptane (meso), etc. C10 has only two types: meso [(3R,4S)-3,4,5-triMe-heptane] and constit., (n-decane, etc.) C11 has three types of isomers: meso [(3R,4S)- 3,4,4,5-TriMeheptane] CTS [(3R,4R)-3,4,4,5-TriMe-heptane] and constit. Alkanes present, beginning with C12, all four types of isomers, e.g. C12: (3R,4R,5S,6S)-3,4,5,6-TetraMe-octane (meso); (3R,4R,5R,6R)- 3,4,5,6-TetraMe-octane (CTS); (3R,4R,5S,6R)-3,4,5,6-TetraMeoctane (diachi), n-dodecane (constit.). The simplest chiral alkane is an isomer of heptane, 3-methyl hexane. Some of alkanes contain four asymmetric carbons and identical ends, similar with linear hexitols or aldaric acids. Therefore, ten chiral linear isomers are possible and some more yet, chiral or achiral, ramified.
As a representative of CnH2n, eicosene can be seen (Figure 1). The first term according to our reasoning is the meso isomer, cis-1,2- dimethyl cyclopropane; CTS isomers are two (trans-1,2-dimethyl cyclopropane) and n-pentene, etc., are constit. Meso, CTS and constit., isomers are also possible for C6H12 (written as 1,2-diMecyclobutane), for C7H14 (written as 1,2-diMe-cyclopentane), for C8H16 (written as 1,3-diMe-cyclohexane). Beginning with C9 (written as 1,2,3,4-tetraMe-cyclopentane), all four types are possible. Four isomers have been presented for dimethyl cyclopropane (C5H10): cis-1,2-diMe cyclopropane (meso), trans-1,2-diMe cyclopropane.
(CTS, two enantiomers), 1,1-diMe cyclopropane (constit.) [103]. According to our systematics, 1-pentene and its linear or ramified isomers are also constit. On a skeleton of cyclopropane, tri-Me derivative (C6H12) presents two types of isomers: meso [1,2,3-triMe cyclopropane (two)] and constit., (1,1,2-triMe cyclopropane, two enantiomers). On a skeleton of cyclobutane, cis-1,2-diMe cyclobutane (meso), trans-1,2-diMe cyclobutane (CTS, two enantiomers) and 1,1-diMe cyclobutane, cis-1,3-diMe cyclobutane and trans-1,3-diMe cyclobutane, all three constit., are presented. We should add for the latter formula other constit., isomers: 1-hexene and its linear or ramified isomers, as well as Me-cyclopentane. 1,2,3,4-TetraMe cyclobutane (C8H16) indicates four meso isomers while 1,1,2,2-, 1,1,3,3-, 1,1,2,3-, 1,1,2,4-tetraMe cyclobutane show only constit., isomers Two CTS should be added: trans-1,2-diEt cyclobutane (enantiomers). For CnH2n-2 (alkynes and alkadienes) meso isomer of heneicosyne (as cis-1,2-dihexyl-3- hexenyl-cyclopropane) is indicated, the first term being C7 (cis-1,2- dimethyl-3-vinyl cyclopropane) or cis-3,5-dimethyl-1-cyclopentene. For monohydroxylic alcohols we have meso isomer of eicosanol (9-hydroxymethyl-8,10-dimethyl heptadecane), the first term is C9 (3,5-dimethyl-4-hydroxy heptane). A general formula represents diols, well exemplified by butane diols. As all the other compounds having two asymmetric carbons only, 2,3-butanediol has but a meso isomer and two CTS; 1,3-butanediol is a constit., isomer. Diols can present diachi isomers only by contribution of alkane chain (see 3,4-diMe-hexane above). Triols, similarly to trimethyl alkanes (see 3,4,5-triMe-heptane) cannot have CTS and diachi isomers, but meso and constit.; the first term is 2,3,4-pentanetriol. Tetrols presents all four types of isomers, the first term is (2R,3S,4R,5S)-2,3,4,5- hexanetetrol. For aldehydes and ketones we introduce meso isomer of heneicosanal (cis-1,2-diheptyl-3-hydroxy-3-butyl), the first term is C5 (cis-1,2-dimethyl-3-hydroxy-cyclopropane).
Meso isomer of eicosanoic acid [cis-1,2-bis(octanol)-3-methylcyclopropane] represents organic acids, and the first term is C3 (cis- 1,2-dihydroxy-cyclopropane). C3 still, as well as C4 and C5 have three types of isomers only (meso, CTS, constit.), while C6 and higher terms possess four (meso of C6 is 1α,2α,3β,4β-1,2-dihydroxy- 3,4-dimethyl cyclobutane). Monoenoic acids are symbolized by a meso isomer of eicosenoic acid [cis-1,2-(7,7’-diheptanol)-3-allylcyclopropane] and the first term is C3 (cis-1,2-dihydroxy-3-allyl cyclopropane). The following isomers are considered constit., isomers of valproic acid (2-propyl pentanoic acid; C8H16O2): 2-ethyl-3-methyl pentanoic acid, di-isopropyl acetic acid, (R)-2- isopropyl pentanoic acid, (S)-2-isopropyl pentanoic acid, octanoic acid [104]. According to our systematics, we have to begin with the finding of a C8H16O2 meso isomer. This can be cis-1,2-dihydroxy- 1,2-diethyl-3-methyl cyclopropane, cis-1,3-dihydroxy-2,2-diethylcyclobutane, 1β,2β,3α,4α-1,2-diethyl-3,4-dihydroxy cyclobutane, or 1β,3β,4α,6α-1,3-dihydroxy-4,6-dimethyl-cyclohexane, or others. As can be seen from their structure, the latter three isomers present also CTS and diachi forms. And the C8H16O2 isomers mentioned earlier, valproic acid inclusively, are all constit. Dienoic acids are made up by the meso isomer of nonadecenoic acid [cis-1,2-(6,6’- dihexanol)-3-butadienyl-cyclopropane] and the first term is C7 [cis- 1,2-dihydroxy-3-(1-butadienyl) cyclopropane].
Biochemical compounds also present meso isomers (Figure1,2). Saturated, mono- and polyenoic fatty acids are represented by the isomers of stearic acid, oleic and eicosapentaenoic acid (the famous omega-3). As is obvious, an isomer of C18H36O2 (cis-1,3-dihydroxycis- 4,6-diheptyl-cyclohexane) present all for type of isomers: meso (cis-1,3-dihydroxy-cis-4,6-diheptyl-cyclohexane), CTS (at least seven pairs of enantiomers) (trans-1,3-dihydroxy-trans-4,6- diheptyl-cyclohexane, etc.), diachi (cis-1,3-dihydroxy-trans-4,6- diheptyl-cyclohexane, etc.) constit., (stearic acid, etc.). A general formula has been elaborated for mono- and polyunsaturated fatty acids (see meso isomers of oleic and pentaenoic acid). For long chain bases (LCB) (sphingosines), LCB d18:1 and LCB d18:0 have been selected. Meso isomers have been also found for LCB t16:0, LCB d16:0, LCB d16:1, LCB t18:0, LCB t18:1, LCB t20:0, LCB t20:1. Squalene presents at least one meso compound. Sterols have been exemplified by cholesterol, sitosterol and digitoxygenin. A similar solution has been found for estrone, stigmasterol, campesterol, ergosterol, C19 (5α-androstanolone), C21 (prednisolone, 11β-Hydroxy-progesterone, pregnenolone, progesterone, corticosterone, cortisol, aldosterone), C24 (biliary acids: cholic, chenodeoxycholic, deoxycholic, lithocholic). Monosaccharides, as a general term, are also represented by meso isomers. Aldotrioses and cetotrioses are illustrated by 1,2,3-trihydroxy-cyclopropane, aldo- and keto-tetroses by 1,2,3,4-tetrahydroxy-cyclobutane, aldoand keto-pentoses by 3-keto-xylitol or pentahydroxy-cyclopentane, aldo- and keto-hexoses by inositols. Nine inositols are known: six meso, one centrosymmetric (scillitol) and two optactive (enantiomers). Of the six meso, five are characterized by 1,4 mirror plane of symmetry, while allo-inositol is devoid of such a plane. Its meso nature ca be explained only by a planar structure, hence the mirror plane of symmetry cuts two opposed bonds. Meso isomers of C6 monosaccharides are also 3-formyl-xylitol and 3-formyl-ribitol, a solution suggested by streptose structure Metzler 2003. Vitamin E is represented by α-tocopherol and α-tocotrienol, but all members of this vitamin have meso isomers, and the same are vitamins K1 and K2. Both meso isomers of vitamin K1 and K2 are indicated. All prostaglandins have a matching meso isomers, as indicated by PGE1, PGF2α, PGE2, PGF3α. A component of coenzyme A, pantoic acid has pentahydroxy cyclohexane as a meso pair.
The planar structure of benzenoid compounds has been successfully used in meso isomers of the following: biopterin (cis-2,4-dihydroxy-3-methyl-3-adenin oxetane), biotin [cis-2,4- dihydroxy-3-methyl-3-(3,4-diamino-thiophene-2)oxetane], adenosin(cis-3,4-dihydroxy-cis-2,5-dihydroxy-1-adenin cyclopentane), FADH2, FMNH2, and even coenzyme A. In order to write meso isomer of FMNH2 we extracted an O atom from a keto bond, however leaving redox system intact. An excellent alternative to this is to link the isoalloxazine system and a phosphonic (not phosphoric!) on C-3 of ribitol. The fact that adenosin possesses a meso isomer indicates that all nucleosides and nucleotides do the same. Meso deoxy-nucleosides and -nucleotides are represented e.g. as cis-1,3-dihydroxy-2-hydroxymethyl-2-adenin cyclobutane or cis-1,3-dihydroxy-2-hydroxy-2-adenin cyclopentane. Camphor is also present. All alkaloids have meso isomers, providing they include a significant aliphatic moiety e.g. piperidine and piperazine. Compounds with a ubiquitous distribution in living matter, the twenty fundamental amino acid are characterized by an unequaled structural variety. However, without any exception, they present meso isomers (Figure 2). Glycine, valine, leucine, isoleucine, proline, threonine, present, besides meso, CTS and constit., derivatives. Lysine and ornitine present all four types of isomers. Amino acids containing an aromatic fragment, or a relative high level of chemical functions are very limited in structural variety. However, we have had again the opportunity to exploit the planar character of benzenoid structures.
AN EXERCISE OF COMPARATIVE CHEMISTRY GIVES AN ANSWER TO AN UNANSWERED QUESTION-WHY IS NATURAL CHEMISTRY AS IT IS?
A question should be raised concerning the hierarchy of the four types of isomers, in other words which of them fills the top place. An intrinsic property of meso combinations is their character of dimerism, hence their molecule is formed of two entities that are contrary in a spatial and optical sense. Of this reason, nine philosophers of ten should declare meso group as being on the top. We ourselves have selected them as structural reference since we thought they have a higher rank than CTS and diachi. Nonetheless that some people could be fascinated by CTS molecules, since they are produced by doubling of the same entity. If we compare the four types, it’s quite obvious that meso, CTS and even diachi are characterized by some structural restrictions. Constit., molecules are characterized by fewer such structural restrictions. Of this reason, probably, natural chemistry opted for them.
When physical chemistry appeared and grew stronger, biologists and other scholars connected with biochemistry, optimistically entertained the hope that physical chemists would discover a marker for natural compounds, as density for gold. Till now such hope never filled, according to our knowledge. Nonetheless, natural combinations possess some unique characteristics, and one of them, in our opinion is the fact that they are less restricted, in structural
sense, than meso, CTS and diachi. A proof for this assertion is the fact that as soon as a living thing dies, nature sends a thousand messengers to recover its component materials. We reckon that at least one of these characteristics is that constit., compounds have a higher number of freedom degrees, in comparison with the other types. It’s a chemical expression of freedom.
In different classes of compounds, a limit has been noticed, and above this limit at least meso isomers are possible, or even all four types. Compounds under this limit have to be considered as primordial. They can reach to the group of combinations producing meso isomers by chemical transformations. Fischer 1890 synthesized a variety C6 monosaccharides from formaldehyde.
СONCLUSION
Atoms or fragments cut by the mirror plane of symmetry are masked (hidden) of polarized light, and what remains, as evidenced by this physical instrument, is a homodimer. All major natural metabolites possessing a significant aliphatic moiety have a meso isomer, hence a dimeric matching. Of the four types of isomers ‒ meso, C2 symmetrical, diastereomeric chiral, constitutional-nature selected constitutional ones, since they are characterized by the highest number of freedom degree.
REFERENCES
- Finar IL (1964) Organic chemistry. Longmans Green and Co Ltd, England.
- Smith, Micheal B, Jerry March (2007) March’s Advanced organic chemistry: reactions, mechanisms, and structure. In: (6th edn), Wiley, USA.
- Britton, Liaaen Jensen G, Pfander S (2004) H Carotenoids, Springer, Switzerland.
- Nicolaou KC, Chen JS (2009) The art of total synthesis through cascade reactions. Chem Soc Rev 38(11): 2993-3009.
- Gao X, Han H, Krische MJ (2011) Direct generation of acyclic polypropionate stereo polyads via double diastereo and enantioselective iridium-catalyzed crotylation of 1,3-diols: beyond stepwise carbonyl addition in polyketide construction. J Am Chem Soc 133(32): 12795- 12800.
- Mamidi N, Gorai S, Mukherjee R, Manna D (2012) Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C. Mol Bio Syst 8(4): 1275-1285.
- Hoffmann RW (2003) Meso compounds: stepchildren or favored children of stereoselective synthesis? Angew Chem Int Ed 42(10): 1096- 1109.
- Wie land T, A Kerber, R Laue (1996) Principles of the generation of constitutional and configurational isomers. J Chem Inf Comput Sci 36: 413-419.
- Fujita S (2016) Half century journey from synthetic organic chemistry to mathematical stereochemistry through chemo informatics. Iranian Journal of Mathematical Chemistry 7(2): 155-221.
- Overman LE, DV Paone, Stearns BA (1999) Direct stereo and enantiocontrolled synthesis of vicinal stereogenic quaternary carbon centers. Total Syntheses of meso- and (−)-Chimonanthine and (+)-Calycanthine. J Am Chem Soc 121 (33): 7702-7703.
- Sugahara T, Yamauchi S, Kondo A, Ohno, F, Tominaga S, et al. (2007 First stereoselective synthesis of meso-secoisolariciresinol and comparison of its biological activity with (+) and (–)-secoisolariciresinol. Biosci Biotechnol Biochem 71(12): 2962-2968.
- Das B, Laxminarayana K, Krishnaiah M, Nandan Kumar D (2009) A Stereoselective total synthesis of verbalactone. Helvitica Chim Acta (92): 1840-1844.
- Wang M, M Feng, B Tang, X Jiang, (2014) Recent advances of desymmetrization protocol applied in natural product total synthesis. Tetrahedron Letters 55: 7147-7155.
- Cahn RS, C Ingold, V Prelog (1966) Specification of molecular chirality. Angew Chem Int Ed Eng 5(2): 385-415.
- Prelog V, Helmchen G (1982) Basic principles of the CIP-system and proposal for a revision, Angew Chem Int Ed Eng 21(8): 567-583.
- Fischer EJ, Hertz Ber (1892) Reduction der schleimsäure. Deut Chem Ges 25(1): 1247-1261.
- Woo S, Keay BA (1996) “SN2’ and “SN2’ like” ring openings of Oxa-ncyclo systems“. Synthesis 7: 669-686.
- Trost, Bary M, Crawley, ML (2003) Asymmetric transition-metalcatalyzed allylic alkylations: Applications in Total Synthesis. Chem Rev 103: 2921-2943.
- Trost BM, DL Van Vranken (1996) Asymmetric transition metalcatalyzed allylic alkylations. Chem Rev 96(1): 395-422.
- Fischer E, Stahel E (1891) Zur Kenntniss der Xylose. Ber Deut Chem Ges 24(1): 528-539.
- Fischer E (1893) Ueber Adonit, einen neuen Pentit. Ber deut chem Ges 26(1); 633-639.
- Lord K (1894) The molecular tactics of a crystal. Oxford, UK: Clarendon Press.
- Kelvin WT (1904) Baltimore lectures on molecular dynamics and the wave theory of light. London Baltimore C J Clay and Sons Publication agency of the Johns Hopkins University.
- Prelog V (2006) Chirality in Chemistry. Chem Acta 79(3): XLIX-LVII.
- Cronin J, Reisse J (2005) Chirality and the Origin of Homochirality.
- Kagan HB, Dang TP (1972) Asymmetric catalytic reduction with transition metal complexes. I. A catalytic system of rhodium (i) with (–)-2,3-(9-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino) butane, a new chiral diphosphine. J Am Chem Soc 94(18): 6429-6433.
- Whitesell (1989) C2 symmetry and asymmetric induction. J K Chem Rev 89(7): 1581-1590.
- Reusch W (2011) Virtual textbook of organic chemistry. Department of Chemistry, Michigan State University USA.
- Kang EJ, E Lee (2005) Total synthesis of oxacyclic macrodiolide natural products. Chem Rev 105(12); 4348-4378.
- Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA (2012) Constructing molecular complexity and diversity: total synthesis of natural products of biological and medicinal importance. Chem Soc Rev 41(15): 5185- 5238.
- Nicolaou KC, Gray DLF (2004) Total synthesis of hybocarpone and analogues thereof. a facile dimerization of naphthazarins to pentacyclic systems. J Am Chem Soc 126(2); 607-612.
- Roberts JD Caserio, MC (1977) Basic Principles of Organic Chemistry. Netherlands.
- Jaeger FM (1917) Lectures on the principle of symmetry and its applications in all natural sciences. Amsterdam Elsevier Publishing Co, Netherlands.
- Vickery HB (1957) Assignment of DL prefixes to the tartaric acids. J Chem Educ 34(7): 339-341.
- Pfaltz A and WJ Drury (2004), Design of chiral ligands for asymmetric catalysis: From C2-symmetric PP- and NN-ligands to sterically and electronically nonsymmetrical PN-ligands. Proc Natl Acad Sci USA 101(16): 5723–5726.
- Pasteur L (1848) Memoire sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire. Compt rend 26: 535-538.
- Kendall J (1953) Great discoveries by young chemists. Th Y Growell Company, USA.
- Derewenda ZS (2008) On wine, chirality and crystalography. Acta Cryst A (64): 246-258.
- Wisniak J Carl Wilhelm Scheele. Rev CENIC Cienc Quim (40): 165-173.
- Svedberg GA (2012) Tribute to the Memory of Carl Wilhelm Scheele (1742-1786) Presented at the 2012 Annual Meeting of the Royal Swedish Academy of Engineering Sciences, Royal Swedish Academy of Engineering Sciences (IVA).
- Hilditch TP (1911) A Concise History of Chemistry D Van Nostr Company USA.
- Van t Hoff JH (1874) A suggestion looking to the extension into space of the structural formulas at present used in chemistry. And a note upon the relation between the optical activity the chemical constitution of organic compounds. Arch Neerland Sci Nat (9); 445-454.
- Le Bel JA (1874) Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc Chim Fr (22): 337-347.
- Hoffmann R, Laszlo P (1991) Representation in Chemistry. Angew Chem (30):1-16.
- Bijvoet JM, Peerdemann AF, Van Bommel AJ (1951) Determination of the absolute configuration of optically active compounds by means of X-rays. Nature (168): 271-272.
- Fischer E (1896) Configuration der Weinsäure. Ber deut chem Ges (29): 1377-1383.
- Klyne W, J Buckingham (1978) Atlas of stereochemistry absolute configurations of Organic Molecules. (2nd edn) london.
- Baer E Fischer HOL (1939) Studies on acetone-glyceraldehyde. IV. Preparation of D- (+)-acetone glycerol. J Biol Chem (128): 463-473.
- Baer E Fischer HOL (1939) Studies on acetone-glyceraldehyde. VII. Preparation of L-glyceraldehyde and L- (–)-acetone glycerol. J Am Chem Soc (61): 761-765.
- Iga DP (2018) Basic principles of the strategy concerning the elucidation of configuration of chiral centers of linear isomeric aldohexoses. Found Chem 20(1): 31-41.
- Fujita S (2016) Chirality and rs-stereogenicity as two kinds of handedness. their aufheben by fujita’s stereoisogram approach for giving new insights into classification of isomers. Bull Chem Soc Jpn 89(9): 987-1017.
- De Pascoli, IC, Nascimento IR, LMX Lopes (2006) Configurational analysis of cubebins and bicubebin from aristolochia lagesiana and aristolochia pubescens. Phytochemistry 67(7): 735–742.
- Jash SK, Brahmachari G (2013) Signpost Biomol Chem (1): 65-168.
- Verotta LF, Elaine E, Tania AA, Nunes J DS (1999) High-performance liquid chromatography-diode array detection tandem mass spectrometry analyses of the alkaloid extracts of Amazon Psychotria species. 841: 165-176.
- Iga DP (2021) Carotenoid Structures, an Illustration of a new kind of symmetry in chemistry. Chemistry Research Journal 6(1): 20-48.
- Cai S, Kong X, Wang W, Zhou H, Zhu T, et al. (2012) Aspergilazine A, a diketopiperazine dimer with a rare N-1 to C-6 linkage, from a marinederived fungus Aspergillus taichungensis. Tetrahedron Lett (53): 2615- 2617.
- Wezeman, Bräse TS, Masters KS (2015) Xanthone dimers: a compound family which is both common and privileged. Natural Products Report 32(1)1-104.
- Gonzalez AG, Martin JD (1971). Taondiol, a new component from taonia atomaria. Tetrahedron Letters 12(29): 2729-2732.
- Gonzalez AG, Martin JD (1972) The synthesis of a taondiol derivative. Tetrahedron letters 13(22): 2259-2260.
- Fischer E, Tafel J (1887) Oxydation der mehrwerthigen Alkohole. Ber Deut Chem Ges. 20(1): 1088–1094.
- Azarnia N, Jeffrey GA, Shen MS (1972) The crystal structures of allitol and d-iditol. Acta Cryst B28: 1007-1013.
- Kull U, Baitinger H (1977) Physiology of ribohexulose (d-allulose) and allitol in itea plants. Pflanzenphysiol Z 82(4): 301-309.
- Li Z, Gao Y, Nakanishi H, Gao X, Cai Let, et al. (2013) Biosynthesis of rare hexoses using microorganisms and related enzymes. Org Chem 12(9): 2434-2445.
- Hassanin HAM, Eassa MAA, Jiang B (2018) Facile synthesis of bioactive Allitol from D-psicose by coexpression of ribitol dehydrogenase and formate dehydrogenase in escherichia coli. Food Bioact 4: 117-122.
- Fischer E, Passmore F (1890) Ueber kohlenstoffreichere Zuckerarten aus d-mannose Ber deut. Chem Ges 23: 569-583.
- Hann RM, Maclay WD, Knauf AE, Hudson CS (1939) Relations between rotatory power structure in the sugar group. XXXI. The Configuration of D-α,α-Mannooctose (D-Manno-L-manno-octose). J Am Chem Soc 61(5): 1268-1269.
- Hudson CS (1941) Emil Fischer’s discovery of the configuration of glucose. J Chem Educ 18(8): 353-357.
- Schmidt RR , Lieberknecht A (1978) Funktionelle D- and L-ribosederivate über eine racematspaltung mit rückführung. Angew Chem 90: 821-822.
- Fischer E, Hirschberger J (1889) Ueber mannose IV. Ber Deut Chem Ges 22(2): 3218-3224.
- Fischer E (1889) Reduction von Säuren der zuckergruppe. Ber Deut Chem Ges 22(2): 2204-2205.
- Fischer Ber E (1890) Ueber die optischen Isomeren des Traubenzuckers, der Gluconsäure und der Zuckersäure. Deut Chem Ges 23(2): 2611- 2624.
- Fischer E (1890) Reduction der säuren der zuckergruppe II.Ber Deut Chem Ges 23(1): 930-938.
- Fischer E (1890) Synthese der mannose und Lävulose. Ber Deut Chem Ges 23(1): 370-394.
- Fischer E (1891) Ueber d und i mannozuckersäure. Ber deut chem Ges 24(1): 539-546.
- Fischer E (1891) Ueber die configuration des traubenzuckers und seiner isomeren. Ber deut Chem Ges 24(1): 1836-1845.
- Fischer E, Fay IW (1895) Ueber Idonsäure, Idose, idit und idozuckersäure. Ber Deut Chem Ges 28(2): 1975-1983.
- Fischer E (1890) Reduction des fruchtzuckers. Ber deut chem Ges 23(2): 3684-3687.
- Fischer E Piloty O (1891) Reduction der zuckersäure. Ber Deut Chem Ges 24(1): 521-528.
- Fischer E (1891) Ueber d und i mannozuckersäure. Ber deut chem Ges 24(1): 539-546.
- Fischer E (1891) Ueber die configuration des traubenzuckers und seiner isomeren. Ber deut chem Ges 24(2): 1836-1845.
- Yang Y, Zhong H, Yao G, He R, Jin B, et al. (2018) Hydrothermal reduction of NaHCO3 into format with hexanehexol, catal. Today 318: 10-14.
- Beck E, Stransky H,Furbringer FEBS Lett M (1971) Synthesis of hamamelose-diphosphate by isolated spinach chloroplasts. FEBS Lett 13(4): 229-234.
- Sellmair J, Beck E, Kandler O, Kress A (1977) Hamamelose and its derivatives as chemotaxonomic markers in the genus primula. Phytochem 16(8): 1201-1204.
- Moore BD, Hackett M Seemann JR (1995) Hamamelitol purification, identification by electrospray ionization mass spectrometry, and quantitation in plant leaves. Planta 195(3): 418-425.
- Pigman W (1957) The Carbohydrates: chemistry, biochemistry, physiology. Academic Press, (ed.), USA.
- Bragg WL, Bragg WH (1913) The diffraction of short electromagnetic waves by a crystal. Proc R Soc London Ser A (89): 248-291.
- Nonell S, Arbogast JW, Foote CS (1992) Production of Fullerene (C60) radical cation by photosensitized electron transfer. J Phys Chem 96(11): 4169-4170.
- Rassat A, László I, Fowler PW (2003) Topological rotational strengths as chirality descriptors for fullerenes. Chemistry A European Journal 9(3): 644-651.
- Finar IL (1963) Organic chemistry, Longmans Green and Co Ltd, UK.
- Pólya G (1937) Kombinatorische Anzahlbestimmungen für Gruppen, Graphen und chemische Verbindungen. Acta Math 68: 145-254.
- Metzler DE, Metzler CM (2003) Biochemistry: the chemical reactions of living cells. Elsevier, Netherlands.
- Nocquet PA (2013) Vers la synthèse d’une nouvelle classe d’iminosucres conformationnellement constraints : ouverture d’azétidines, cyclisation 4-exo-trig et C-H amination catalytique. Autre. Université de Strasbourg Français. NNT: 2013STRAF047.
- Pfaltz A (1996) Design of chiral ligands for asymmetric catalysis: from C2-symmetric semicorrins and bisoxazolines to non-symmetric phosphinooxazolines. Acta Chemica Scandinavica 50: 189-194.
- Ghosh A K, Mathivanan P, Cappiello J (1998) C2-Symmetric chiral bis(oxazoline)-metal complexes in catalytic asymmetric synthesis. Tetrahedron Asymmetry 9(1): 1-45.
- Bach RD, Dmitrenko O (2002) The effect of geminal substitution on the strain energy of dioxiranes. the origin of the low ring strain of dimethyldioxirane. J Org Chem 67(11): 3884-3896.
- Ellis AV, Kannangara GSK, Wilson MA (2003) Chemistry of sodium lactate formation under simulated alumina refinery conditions. Ind Eng Chem Res 42(14): 3185-3189.
- Wilson MA, Kannangara GSK, Ellis AV (2004) Carbohydrate rearrangements in humic solutions. In Combined national conference of the Australian Organic Geochemists and the International Humic Substances Society, 16-19 February, Blue Mountains, New South Wales, Australia.
- Mendkovich AS, Leibzon VN, Mairanovski SG, Krayushkin MM, Klimova TA, et al. (1978) Electroreduction of polyhedrane derivatives 1. Structural effect of keto derivatives of bicyclo [3.3.1] nonane, adamantane, and noradamantane on electrochemical reduction. Russ Chem Bull (27): 1639-1643.
- Steiner GW, Strobel GA (1971) Helminthosporoside, a host-specific toxin from helminthosporium sacchari. J Bio Chem 246: 4350-4357.
- Skrela BC (2012) Synthesis and coordination chemistry of new multidentate ligands for applications in olefin polymerization and dinitrogen activation. A Thesis Submitted to the Faculty of Graduate Studies in Partial Fulfilment of the Requirements, for the degree of Master of Science graduate program in chemistry, New York University, USA.
- Gupta RR, Kumar M, Gupta V (1998) Four-membered heterocycles. In: Heterocyclic Chemistry. Springer, Berlin Heidelberg Heterocyc Chem 357-410.
- Bull J A, Croft R A, Davis OA, Doran R, Morgan KF, et al. (2016) Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem Rev 116(19): 12150-12233.
- Balaban AT (1978) Chemical graphs XXXII constitutional and steric isomers of substituted cycloalkanes. Croatica Chemica Acta CCACAA 51(1): 35-42.
- Shimshoni JA, Bialer M, Wlodarczyk B, Finnell RH Yagen B (2007) Potent anticonvulsant urea derivatives of constitutional isomers of valproic acid. J Med Chem 50(25): 6419-6427.
Article Type
Review Article
Publication history
Received Date: May 05, 2022
Published: September 14, 2022
Address for correspondence
Dumitru Petru I Iga, University of Bucharest, Romania
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Dumitru Petru I Iga. All the Major Metabolites Containing a Significant Aliphatic Moiety Possess At Least One Real or Envisaged Meso Isomer. 2022- 4(5) OAJBS.ID.000486.