African Children in Danger from High Levels Organochlorine Pesticides in Blood and Urine Samples
ABSTRACT
Despite reported effects and synergistic harm, organochlorine pesticides (OCPs) are less studied in Africa children. Therefore, concentrations of eight OCPs in blood and urine of children within Owerri municipality were determined. Thirty-six (36) blood and urine samples were collected from children aged 4-14 years. Concentrations were determined using 6890 GC systems coupled with 5971 mass selective detector. 1μl sample was loaded in a split less mode and the GC helium with carrier gas had at temperature of 80oC to 180oC and finally to 300oC. Result shows that Aldrin (45233±65473ppm) was the highest while mirex (38±5.099ppm) was lowest in blood of children. In urine Aldrin (193±22.84ppm) was highest and P’P’DDD (4.56±4.90ppm) was lowest. P’P’DDD was not detected in children at UPS, WCP, CSO and HEO. Therefore, both blood and urine concentrations of OCPs had no significant difference (P<0.05) between schools. Elimination ratios were highest for DDT (20.390 at MNO. Linden (5.11) at MNO was amongst the lowest ER. In blood, Pearson correlation coefficient was highest for DDT-DDD (0.612), DDT-methoxychlore (0.599), DDD-methoxychlore (0.658) and aldrine-methoxychlore (0.840) and methoxychlore-chlordane (0.750). OCPs in the urine showed low correlation with each other. Principal component analysis (PCA) plot in rotated space for OCPs in blood and urine revealed two major clustering in OCPS in each group highly correlated. The strong association amongst OCPs in each group could be an indication of cochemical toxicology effects. Regression analysis revealed poor limpidity between OCPS in blood and urine except P’P’DDT. Therefore, some OCPs in blood may not show similar concentrations in urine. The questionable concentrations of OCPs in blood and urine are a serious call for concern.
KEYWORDS
Congeners; Dirty dozen; Metabolites; Pesticide residue; Sequelae; Xenobiotics
ABBREVIATIONS
OCPs: Organochlorine Pesticides; PCA: Principal Component Analysis; TILT: Toxicant Induced Loss of Tolerance; SRI: Sensitivity Related Illness; LGAs: Local Government Areas
INTRODUCTION
Dangers faced by African children have been overlooked as regards exposure to pollutants from their environment [1,2]. African children are more exposed to xenobiotics because of factors such as low literacy and less enforcement of environmental laws and standards. In some countries, these standards and laws are even completely unknown and absent. Most often the leaders are semiliterate and see no need to invest in the health and healthy environment for the children. Beyond the question of costs and benefits is an ethical issue that society must also consider. Children have the right to realize their full potential and when a society allows its children to be exposed to pollutants unchecked it is directly denying the children of that right [3,4]. Organochlorine pesticides (OCPs) are a group of synthetic chlorinated hydrocarbon compounds that have been widely used globally in the agricultural and chemical industries. They exhibit high bioaccumulation, slow degradation and toxicity at even low concentrations. Despite the ban at Stockholm convention, developing nations still experience vast applications of these chemicals [5,6] and some still use them for pest control, and worst of all for fishing as is the case in some part of Nigeria.
There exists extensive literature on neurodevelopment, neurologic and effects to humans arising from chronic low-level exposures to OCPs [7-9]. Research showed a strong relationship between low concentrations of OCPs and type 2 diabetes [10,11]. There is equally substantial evidence that OCPs interact with endocrine systems through estrogen and androgen receptors [12]. OCPs such as lindane, chlordane is linked to hematological disorders and anemic conditions [13,14]. When absorbed for longer periods, OCPs can cause enzyme inducement, a situation that causes metabolic disorder. OCPs act chiefly by acute toxic activity on the central nervous system. There OCPs can cause hyperexcitable brain, a situation that ends up in convulsion or myoclonic jerking, paresthesia’s and tremor [15]. OCPs in children are responsible for carcinogenic tumors, and neurotoxity psychiatric sequalae reduced cocongritiue development and behavioral disorders and depression [16]. Xenobiotics have become an issue of concern with exponential growth of evidence are currently being exposed to many types of toxicants [17]. OCPs fall in this category of toxicants and exert their toxicity in many ways leading to pathophysiological mechanisms such as damage to mitochondria, oxidative stress, apoptosis and epigenetic modifications [18]. OCPs have also been known to exhibit synaptic dysregulation Hille 1968. Recently the ‘toxicant induced loss of tolerance’ (TILT) has been proposed as another potential way of harm from OCPs. When in the presence of other toxicants or chemical agents OCPs can function synergistically, to harm the organism. This could lead to a multisystem reaction called ‘sensitivity related illness’ (SRI) [19,20].
According to the Centre for Disease Control pollutants now cause accrual in adult and children CDC 2013 and Health Canada has reported stockpile of toxicants within the populace [21]. There are equally reports of nonvolatile OCPs trapped in aerosols or dust particles and when swallowed by adults or children could end up being absorbed in the intestine. After many years of being banned it is expected that OCPs will be on a decrease in the environment. However, researchers have shown that OCPs are continually being found in the environment and human bodies due to international travel, ongoing use of pesticides in mostly third world countries, globally exchange of food from areas where OCPs are used to areas where use has been discouraged [3] and slow rates of degradation in the environment. Being lipophlic OCPs have lower vapor pressure and penetrate easily though the gut, lungs and skin. These characteristics make them easily adsorbed on skin, gut, lungs and inhalation. Chemically OCPs fall into six groups in terms of structures;
(i) DDT and endogues
(ii) Hexachlorobezene
(iii) Hexachlorocydolexane
(iv) Cyclodine
(v) Chlordecone
(vi) Toxophene [22].
Another grouping criterion of OCPs could be their ability to be absorbed across the skin which increases their presence in blood. OCPs such as HCH, Lindane, aldrien, dieldrin and heplachlore show high dermal efficiency and belong to the first group. The second group consist of DDT and analogues, methoxychlore and mirex which all show low dermal absorption.
Many researchers employ gas-liquid chromatography to identify and quantity OCPs in blood samples within few days of pesticide exposure. DDT, dieldrine, mirex, heptachlore, chlorodane are known to persist the in tissue and blood for months while others may be excreted from the body within days. This reduces detection and so blood levels show more correlation to acute toxicity unlike adipose tissue concentrations which correlate better with historic exposure. Urinary measurements of metabolites of some OCPs could be employed in characterizing occupational exposure [23]. The U.S. EPA has curtailed the availability of OCPs while some countries continue to use it. Even those thought to have less toxic effects like lindaine are no longer recommended by the American academy of pediatrics while some states like California have banned OCPs entirely. Clinically, it is difficult to assess and manage persons with xenobiotics related health issues. Rather than remaining in blood, many OCPs are sequestered and accumulated in tissues. For these reasons, some researchers have opined that biomonitoring of OCPs in blood and urine may not reflex actual levels in the body. Elimination through induced sweat and re-absorption in the gastrointestinal systems are equally some ways by which actual concentrations of OCPs in blood may not be measured. However, it is shown that OCPs concentrations associated with various health problems and many epidemiological studies have shown the strong relationship between OCPs and illness. Therefore, there is a need to at least have data of OCPs in children. This could indicate the need for stringent rules to reduce the risk to children. In addition, such studies have not been conducted in Owerri. There exists a myriad of sources from which these OCPs can enter our children ranging from low sanitation to poor carriages, liberal use of pesticides and the importation of food stuffs from various part of the country into Owerri such as rice, beans, groundnut, yams and tomatoes, oranges [24] all associated with pesticides. The aim of this research was to determine the concentrations of a group of OCPs commonly used organochlorine pesticides in blood and urine of children from selected primary schools in Owerri municipality. OCPs here include DDT, DDD, lindane, aldrien, dieldrine, methoxychlore, heptaclore and mirex. Current study could make available such information that could give policy makers and create awareness on the need to formulate policies that would reduce the body burdens of xenobiotics in children.
METHODOLOGY
Description of the Study Location
Owerri metropolis, lies within latitude 5.48o North and longitude 7.03o Imo State, southeast Nigeria. This area falls within the heart of the humid African tropical region. Owerri metropolis lies within one of the three local government areas (LGAs) that make up Owerri city, the capital of Imo state of Nigeria set in the heart of the Igbo land. Playgrounds were designated with the letters of the words in the name of the school. Out of 25 government schools within Owerri metropolis, 9 schools selected and thus were used in this study. Playgrounds were selected to reflect spatial variability and traffic/commercial influence associated with each zone and differences in land use within an urban setting. Samples will be collected from schools; along busy roadway/highway, within residential, commercial and industrial areas, along a zone where industries and factories are located [25].
Participant demographics: 36 children residing in Owerri municipal were recruited as follows; 18 boys and 18 girls. Child participation was limited to those aged 4-14 years because that was the age for most children using playgrounds at selected schools. Child participant was checked for any differences between age and gender using p-values. Children were 50% male and 50 % were male and sample collected lasted for two weeks.
Human subjects interactions: Approval was sought and obtained from the Imo State University ethics committee and permission sought from the parents with written informed consent obtained from each mother for her child’s participation, along with the child’s assent. Participants were met either at their school and it was established that they frequent the playgrounds. All children were confirmed to come from low-income homes, as it was confirmed that all children frequenting the playgrounds were from poor backgrounds.
Surveys and biological samples: A written survey was filled out by each mother to capture diet, self-reported health, activity, and household information. Mothers were asked to provide the same information for their children. Fish consumption, with species, over the most recent six months was addressed along with consumption of local produce and game. Participant recollection was reinforced by a 24-hour recall survey. From children, biological samples were collected for analysis. Participants were instructed to give a midstream urine sample into acid-washed 120 mL BD Vacutainer urine cups (urine reflects exposure to inorganic mercury). Intravenous blood samples were collected following sterile procedures by a licensed phlebotomist from the antecubital fossa into BD Vacutainer. Blood samples were analyzed for whole blood analyzed directly after being vortexed. All samples were stored at -20 °C in a locked freezer at the Imo state university [26;27] prior to analysis.
DETERMINATION OF OCPS
Instrumentation
The OCPs of interest were analyzed using a 6890 GC system coupled with a 5971-mass selective detector (Agilent Technologies). Chromatographic separation of the components was done using a capillary column (30 m x 0.25 mm internal diameter, 0.25 μm film thickness) HP-5MS and helium as the carrier gas flowing at 1.5 mℓ/min–1. Conditions of gas chromatography separation were as follows: injector temperature was set at 250 °C, initial column temperature was at 70 °C and held for 0.5 min. This ramped at 25 °C∙min–1 to 150 °C. It was then ramped at 30°C.min–1 to 200 °C. This further ramped at 8 °C∙min–1 to 280 °C and held for 20min. Detection of the separated OCPs was achieved using a GC/MS system operated in selected ion monitoring mode with the electron impact ionization set at 70eV. The temperatures of the ion source, transfer line and the quadrupole were held at 230 °C, 280 °C and 150 °C, respectively. Quantization of the residues was accomplished using a 7-point standard calibration curve in the concentration range of 0 to 1000 ng/ℓ–1. GC-MS was used for indicator and OCPs analysis. 6890N GC (Agilent, USA) equipped with a 60m x 0.25mm x 0.25 μm DB5-MS column (Agilent J&W, USA) coupled to Quattro Micro GC-MS (Waters, Micromass, UK) operated in EI+ was used; at least 2 MRM transitions was recorded for each compound analyzed. Injection was done in split less mode at 280°C and 1 μℓ sample loaded. Helium gas was used as carrier gas at the flow of 1.5 mℓ/ min-1. The GC temperature was programmed at 80 °C (1-min hold), then 15 °C∙min–1 to 180°C, and finally 5 °C∙min–1 to 300 °C (5-min hold) [28]. Raw data was processed using Target Lynx soft- ware Waters, Micromass, UK [9].
Statistical Analysis
In order to retain maximum interpretability, data was analyzed and reported without transformations. Statistical analyses were performed in SPSS version 18.0. Using elimination ratios principal component analysis, regression and correlation analysis was interpreted. Primary comparisons of interest were differences between schools and amongst children as well as comparison to benchmark values as seen throughout literature. Coefficient of variation (CV %) was calculated using the equation described by [29]. Variation was categorized as: CV % less than 20 as little variations; CV % between 20 to 50 as moderate variation and CV % greater than 50 as high variations. Analysis of variance (one-way ANOVA): ANOVA was employed for the purpose of comparing mean metal concentrations among the playgrounds and statistically significant differences were described when P < 0.05 [30].
Quality Control and Quality Assurance
Analytical grade chemicals and reagents purchased from FinLab Owerri were used without further purification. These include nitric acid (HNO3) 6.5% v/v HCl, sodium sulphate and potassium hydrogen carbonate which all purchased from Merck through FinLab agents while double distilled water used throughout the analysis; working standard of OCPs for references sourced from Fluka (Buch’s, Switzerland).
RESULTS AND DISCUSSION
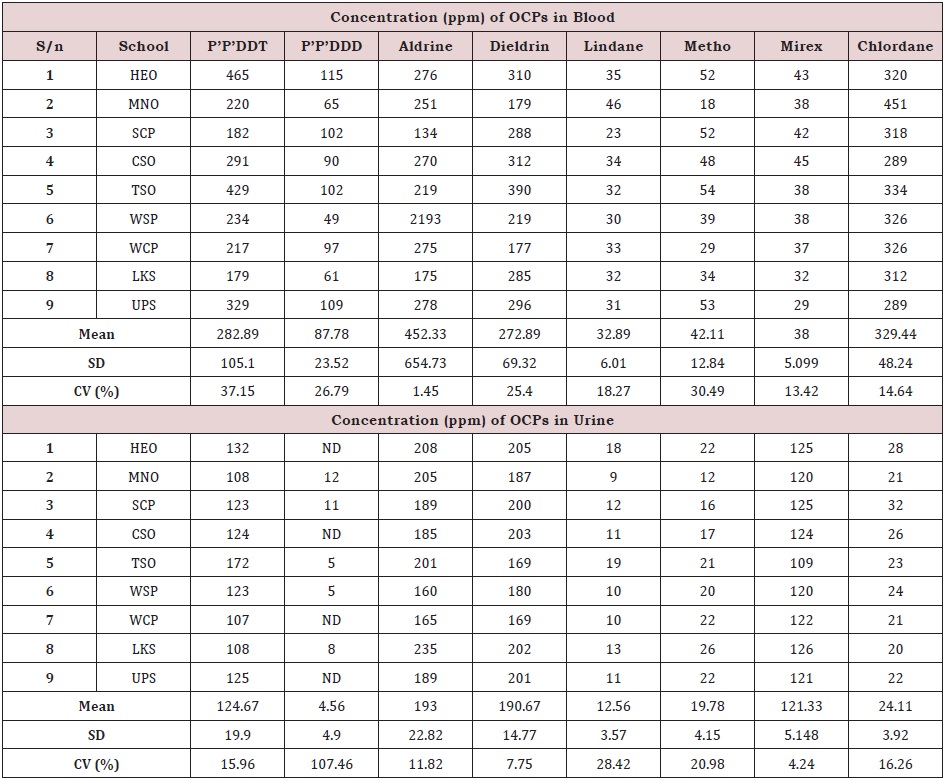
The concentration of OCPs analysis including P’P’DDT, P’P’DDD, aldrine, dieldrin, lindane, methoxychlore, mirex and chlordanein whole blood and morning urine sample from different schools are presented in Table 1. In blood, the concentration of DDT ranged from 179ppm at LKS to 465ppm at HEO with mean of 282.89±105.10ppm. DDD concentrations ranged from 49ppm at WSP to 115ppm at HEO with mean of 87.78±23.52ppm. Aldrine concentrations ranged from 134ppm at SCP to 2193ppm at WSP with mean of 452.33±654.73ppm. Dieldrin concentrations ranged from 177ppm at WCP to 390ppm at TSO with mean of 272.89±69.32ppm. Lindane concentrations ranged from 23ppm at SCP to 46ppm at MNO with mean of 32.89±6.01ppm. Metho concentrations ranged from 18ppm at MNO to 54ppm at TSO with mean of 42.11±12.84ppm. Mirex concentrations ranged from 29ppm at UPS to 43ppm at HEO with mean of 38±5.10ppm while chlordane ranged from 289 at CSO to 451ppm at MNO with mean of 329.44±48.24ppm. In order of decreasing OCPs concentration; mean values revealed; Aldrin> Chlordane > DDT > Dieldrin> DDD >Metoxychlore>Lindane>Mirex, DDT had the highest value in children’s blood at HEO (465ppm) and TSO (429ppm) while methoxychlore was lowest.
The concentrations obtained in the current study were very high when compared to other related studies [31] detected only chlorpyrifos (0.009ppm) and pyributicarb (0.001ppm) in the blood samples of villagers involved in pesticide application at District Vehari (Punjab), Pakistan. Similarly, [32] reported very low concentrations of OCPs for blood compared to the current study. The high concentrations of blood-OCPs reported in this study are probably due to the high exposure to OCP in the environment, air, foods and water [33,34]. Generally, the high concentrations of DDT and DDD in blood are probably due to the fact that the urbanized environment have more of these pollutants in the air and can be absorbed through the gut and lungs during inhalation. Foods such as fish and tomatoes have been found to have these OCPs in them and it has been observed that city dwellers consume large quantities and are likely to take in these pollutants. The OCPs concentration in blood had low to moderate variations (Table 1). The concentrations of OCPs in the blood showed significant differences (p< 0.05) while between schools the concentrations showed no significant difference (p > 0.05).
In urine, the concentration of DDT ranged from 107ppm at WCP to 132ppm at HEO with mean of 124.67±19.90ppm; DDD was not detected at WCP, HEO, CSO and UPS but was 12 ppm at MNO with mean of 4.56±4.90ppm. Aldrine had a range of 160ppm at WSP to 235ppm at LKS with mean of 193±22.82ppm. Dieldrin had a range of 169 at TSO and WCP to 205ppm at HEO showing mean of 190.67±14.77ppm. Lindane ranged from 10ppm at WCP and WSP to 19ppm at TSO with mean of 12.56±3.57ppm. Metho ranged from 12 ppm at MNO to 26ppm at LKS with mean of 19.78±4.15ppm. Mirex ranged from 109ppm at TSO to 126ppm at LKS with mean of 121.33±5.15ppm while chlordane ranged from 20ppm at LKS to 32ppm at SCP with mean of 24.11±3.92ppm. The concentrations obtained in the current study were very high when compared to other related studies. [23] studied OCPs residue in urine of male and female sample from El-Hosh town community, South Gezira and detected only DDE in the mean range of 4.18 to 5.17ppm. Similarly, [35-40] reported very low concentrations for urine compared to the current study. The high concentrations of urine-OCPs reported in this study are probably a reflection of the high concentrations in the blood of the children studied. In decreasing order, the concentrations of OCPs in urine were Aldrin > Dieldrin > DDT >Mirex> Chlordane > Lindane > DDD. While Adrien (208ppm) was highest amongst OCPs in children urine, DDD (5ppm) was lowest and in some DDD was not detected. Even at low concentrations OCPs are disease initiating agents in human body [10]. (Table 1) shows that the variation of OCPs concentration in blood (aldrin; 1.45%) ranged from low to moderate variation (P’P’DDT; 37.15%) while variations of OCPs concentrations in urine ranged from low variation (mirex; 4.24%) to high variation (P’P’DDD; 107.46%). The concentrations of OCPs in the urine showed significant differences (P<0.05) while between schools the concentrations showed no significant difference (p > 0.05).
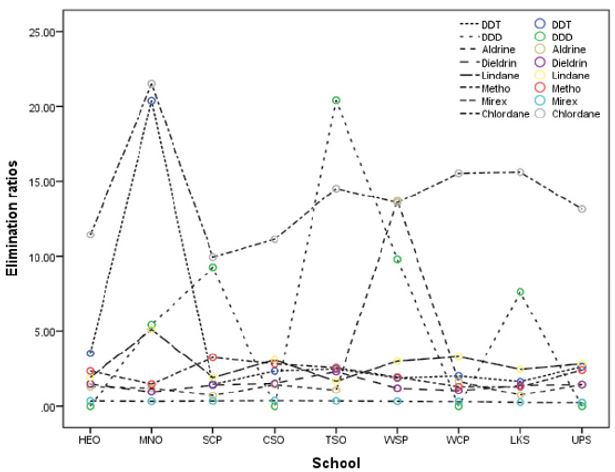
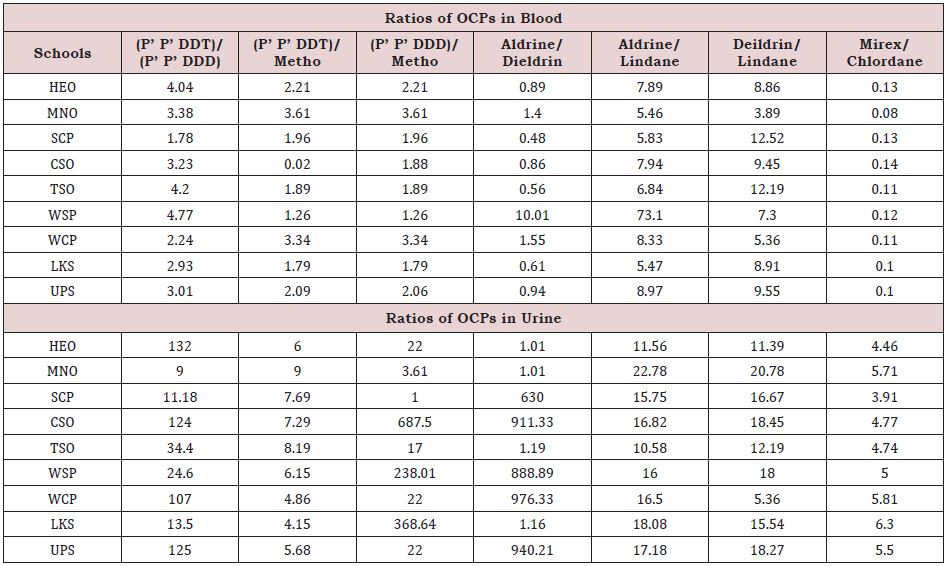
Ratios of OCP concentrations in blood to OCP concentrations in urine were computed and presented in (Figure 1) as elimination ratios. Large values of ratios are indicative of low excretion of the OCP in urine and thus a dangerous pointer, while low values of ratios are a pointer of good removal of OCP in urine. Recall that many excretion routs exist for OCP in human body including sweat and feces. In generally, large values of ratios were obtained except for mirex in all schools, dieldrin at MNO, Aldrin at SCP and LKS while DDD had zero at HEO, CSO and UPS due to no detection of OCP in urine indicating a harmless situation. The ratios for DDT at MNO (20.37); DDD at MNO (5.42), SCP (9.27), TSO (20.40), WSP (9.80) and LKS (7.63); Aldrin at WSP (13.71); lindane at MNO (5.11) and chlordane in all schools were considered very high and therefore harmful conditions may prevail. The overall large values could be due to enterohepatic reabsorption and affinity to adipose tissue and so some types of OCPs are not efficiently eliminated from the human body and may accrue in tissues [40-45].
Correlation of Metals in Blood and Urine
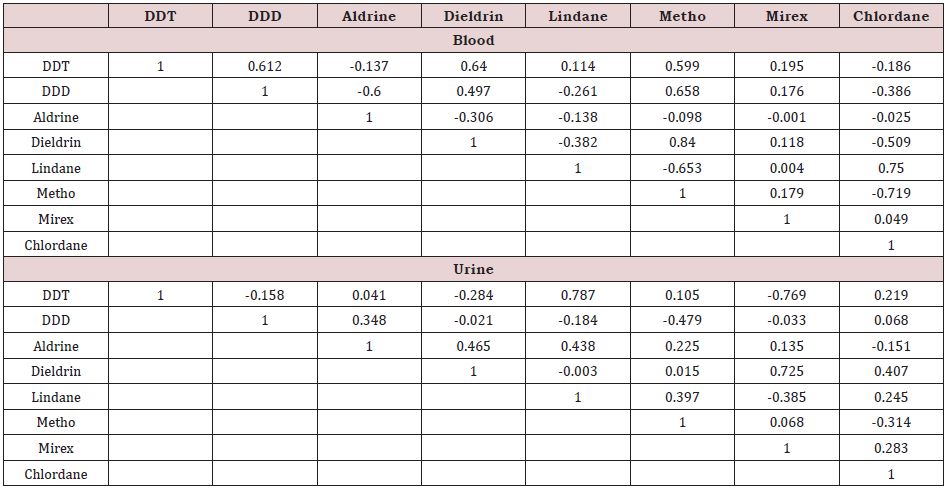
Pearson’s correlation coefficients were used to describe the relations between the studied OCPs in the blood and urine. The computed Pearson’s correlation coefficients are presented in (Table 2). Significant positive correlation was observed among the following pairs of OCPs in blood: DDD with DDT and Aldrin, metho with DDT, DDD and dieldrin, chlordane and lindane. These strong positive correlations found between the OCPs studie indicate eventual interactions between them. This can probably indicate increase in their toxic effects in children even at a low environmental level of exposure [46-56]. Meanwhile in urine only mirex-dieldrin (0.725) and lindane-DDT (0.787) showed strong correlations. Overall, in urine the OCPs showed low correlations.
Principal Component Analysis
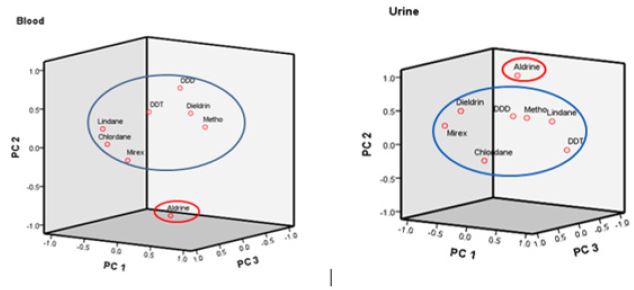
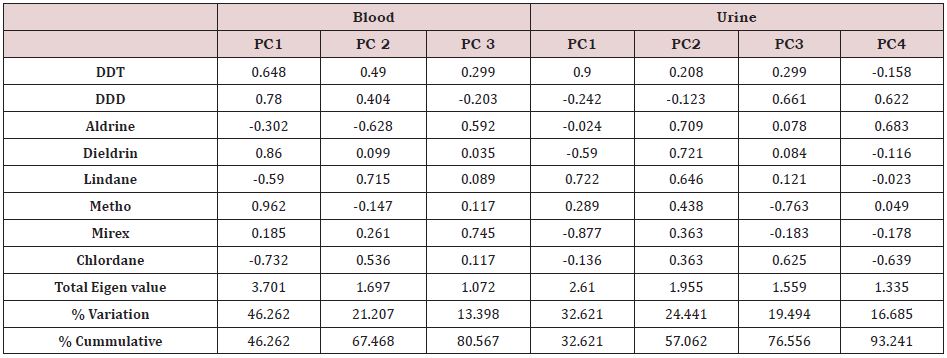
A Principal component analysis (PCA) plot in rotated space for OCPs in blood and urine are presented in (Figure 2) and shows two major clustering of the OCPs into two different groups viz the Aldrin group and other studied OCPs. The OCPs in each group are highly correlated with each other. The principal components were extracted with an eigen value of >1, and the variances for the first (PC1), second (PC2) and third (PC3) groups were 46.26%, 21.21% and 13.39 % respectively for blood while for urine PC1, PC2, PC3 and PC4 were 32.62 %, 24.44 %, 19.49 % and 16.69% respectively (Table 3). Based on the PCA for blood, DDT, DDD, dieldrin and methoxychlore were in a particular group (PC1), lindane and chlordane were in a different group (PC2) while mirex and aldrine are in PC3. OCPs in a given group are seen to have strong association and could indicate co-chemical toxicological effects. Based on the PCA for urine, the OCPs; DDT and lindane were in a particular group (PC1), aldrine, dieldrine and mirex were in a different group (PC2), in PC3, DDD and chlordane while again in PC4 DDD were grouped with aldrine.
Regression Analysis
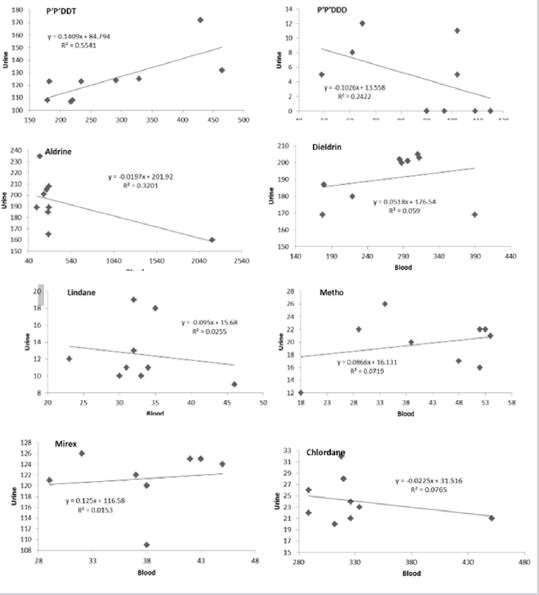
Linear regression analysis was done to evaluate relationships between the concentration of OCP in blood and urine of children. The relationship is presented in (Figure 3) showing the R2 values and regression equations. The linear regression provides important information on association between the OCPs variables in the two media (i.e., blood and urine). The extent of association is measured on a scale 1 (perfect positive relationship), 0 (no relationship) and -1 (perfect negative relationship) [56-60]. The regression, although positive is poorly linear with r values less than 0.5 except for DDT. Therefore, suggests that the concentrations of OCPs in blood are not totally responsible for these in urine. Some researchers have suggested that OCPs could find their way in the bodies of children in many ways including adsorption through the skin.
In this way a child exposed to pollutants in soil during play may show a different concentration in the morning and another in the evening. These high concentrations may not be indicative of body burdens of these OCPs because their ADMET are different [61- 64]. In addition, various OCPs are up taken into children through such means as inhalation, ingestion, vertical transmission and transdermal. This could be reflected as a difference in both blood and Urine levels of OCPs. Despite the ban on DDT and DDD were higher in both blood and urine of children studied. In order to reduce or exposure, the rations of certain OCPs were calculate and used to estimate the length of existence in the blood, generally small values of OCP ratios indicate long term exposures [65], while values greater than 1 indicate fresh exposure (Table 4) shows that DDT/DDD, DDT/METHO, DDD/METHO had values greater than 1 and could be assumed to have been from freshly exposed sources of contamination in children’s blood. On the order hand ratios for aldrine/dieldrine and mirex/chlordane were generally less than1, implying that the concentration of these OCPs in blood are from long term exposure. Other ratios were observed to be much greater than 1, indicating a fresher exposure. In urine ratio of OCPs were generally much greater than I compared to those ratios for blood. It was observed that OCPs ratios in urine were attributable to fresh exposure (Table 4).
CONCLUSION
This research has successfully determined and reported concentrations and distribution of eight OCPs in blood and urine of native African children living and attending selected schools within Owerri municipality. This work is perhaps the first of its kind. There could be risk arising from these concentrations given that these pollutants have typical characteristics of being bio accumulative. There is close association amongst OCPs studied and that suggests co-chemical toxicity, though concentrations in blood showed little influence on those of the urine. Following the presence of questionably high concentrations of these OCPs in blood and urine of African children, they may continuously be exposed to both lowand high-level exposure of these toxic pollutants. OCPs ratios reveal that most of the pollutants were from fresh sources of exposure and therefore exposure routes need to be quickly identified and eliminated while measures put in place to monitor OCPs in playgrounds, water, and food. This work has call for measures to reduce OCPs concentrations in children’s blood from Owerri municipal. Same can be said for most of African children in urban areas.
ACKNOWLEDGEMENTS
Authors greatly acknowledge the expertise of Enyoh Christian Ebere, Okechukwu Stela-Maris and Amaobi Colins for immense support during sample collection, laboratory analysis and statistical analysis.
AUTHORS CONTRIBUTIONS
AWV initiated the study ideas, design sampling, collected prepared the consent letter and meet the head teachers and parents, and helped perform the analytical determinations, acquired and analyzed data and contributed to the writing of the manuscript and revision. AW organized the experimental setting, supervised the work, and wrote the manuscript. ACM proofread the manuscript and added to the discussion. LKC arranged references, read the final work and was instrumental in obtaining consent from parents and children.
FUNDING
The research was fully funded from grant awarded by TETFUND research project intervention, second batch 21- 2014-IBR-IMSU-17.
REFERENCES
- Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40(1): 241-258.
- Yasunaga S, Nishi K, Nishimoto S, Sugahara T (2015) Methoxychlor enhances degranulation of murine mast cells by regulating FceRImediated signal transduction. J Immunotoxicology 12(3): 283-289.
- Garry VF (2004) Pesticides and children. Toxicol Appl Pharmacol 198(2): 152-163.
- Charnley G, Putzrath RM (2001) Children’s health, susceptibility and regulatory approaches to reducing risks from chemical carcinogens. Environ Health Perspect 109(2): 187-192.
- ATSDR (Agency for Toxic Substances and Disease Registry) (2009) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). Case studies in environmental medicine. United States Department of Health Services, Public Health Service, Atlanta, Georgia USA.
- (2016) United States Environmental Protection Agency DDT: A briefhistory and status.
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V (2009) Organochlorine exposure and incidence of diabetes in a cohort of great lakes sport fish consumers. Environ Health Perspect 117(7): 1076-1082.
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez Ramos JR (1994) Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol 36(1): 100-103.
- Heusinkveld HJ, Westerink RHS (2012) Organochlorine insecticides lindane and dieldrin and their binary mixture disturb calcium homeostasis in dopaminergic PC12 cells. Environ Sci Technol 46(3): 1842-1848.
- Son HK, Kim SA, Kang JH, Chang YS, Park SK, et al. (2010) Strong associations between low-dose organochlorine pesticides and type 2 diabetes Korea. Environment International 36(5): 410-414.
- Genuis SJ (2006) The chemical erosion of human health: adverse environmental exposure and in-utero pollution-determinants of congenital disorders and chronic disease. J Perinat med 34(3): 185-195.
- Upson K, De Roos AJ, Thompsonetal ML, Sathyanarayana S, Scholes D, et al. (2013) Organochlorine pesticides and risk of endometriosis: findings from apopulation-based case-control study. Environ Health Perspect 121(11-12): 1319-1324.
- Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, et al. (1998) Environmental endocrine disruption: an effects assessment and analysis. Environmental Health Perspec 106(Suppl 1): 11- 56.
- Benarbia MEA, Macherel D, Faure S, Jacques C, Andriantsitohaina R, et al. (2013) Plasmatic concentration of organochlorine lindane acts as metabolic disruptors in HepG2 liver cell line by inducing mitochondrial disorder. Toxicologyand Applied Pharmacology 272(2): 325-334.
- Genuis SJ, Kelln KI (2015) Toxicant exposure and Bioaccumulation: a common and potentially reversible cause of cognitive dysfunction and dementia. Behav Neurol.
- Jurewicz J, Hanke W (2008) Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. Int J Occup Med Environ Health. 21(2): 121-132.
- Bouwman H (2003) POPs in South Africa: The handbook of environmental chemistry. Persis Org Pollut 30: 297-320.
- Pathak R, Suke SG, Ahmed T, Ahmed RS, Tripathi AK, et al. (2010) Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Human &Experimental Toxicology 29(5): 351-358.
- Genuis SJ (2010) Sensitivity-related illness: the escalating pandemic of allergy, food intolerance and chemical sensitivity. Sci Total Environ 408(24): 6047-6061.
- Genuis SJ (2014) Pandemic of idiopathic multimorbidity. Canadian Family Physician 60(6): 511-514.
- (2015) Human biomonitoring of environmental chemicals. Health Canada
- Genius JS, Lane K, Birkholz D (2016) Human elimination of organochlorine pesticides: blood, urine and sweat study. Biomed research international.
- Taha MEM, El-Zorgani GA, El-Hassan AM, Salghi R (2013) Evaluation of organochlorine pesticide residues in human urine from rural population in sudan. J Mater Environ Sci 4(6): 987-992.
- Fernandez M, Pico Y, Manes J (2001) Pesticide residues in oranges from valencia (Spain). Food Addit Contam 18(7): 615-624.
- Verla AW, Ngozi Verla E, Medo Ajero C, Chioma Lele K, Stella Marris NO, et al. (2019) Biomonitoring of heavy metals in blood and urine of African children from Owerri metropolis, eastern Nigeria. J Chemical Health Risks 9(1): 11-26.
- Domínguez-Cortinas G, Díaz-Barriga F, Martínez-Salinas RI, Cossío P, Pérez-Maldonado IN (2013) Exposure to chemical mixtures in Mexican children: high-risk scenarios. Environ Sci Pollut Res Int 20 (1): 351-357.
- Doong RA, Liao PL (2001) Determination of organochlorine pesticides and their metabolites in soil samples using headspace solid phase microextraction. J Chromgph 918(1): 177-188.
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, et al. (2011) Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. ploS one 6(1).
- Mbah CN, Anikwe MAN (2010) Variation in heavy metal contents on roadside soils along a major express way in Southeast Nigeria. New York Science Journal 3(10): 103-107.
- Khizar H, Ashfaq M, Ashfaq U, Saleem MA (2010) Determination of pesticide residues in blood samples of villagers involved in pesticide application at District Vehari (Punjab), Pakistan. African Journal of Environmental Science and Technology 4(10): 666-684.
- Genuis SJ, Genuis RA (2016) Preconception care: a new standard of care within maternal health services. Bio Med Research International.
- Perez-Maldonado IN, Trejo-Acevedo A, Pruneda-Alvarez LG, Gasper-Ramirez O, Ruvalcaba-Aranda S, et al. (2013) DDT, DDE and 1-hydroxyperene levels in children(in blood and urine samples)from Chiapas and Oaxaca, Mexico. Environ Monit Assess 185(11): 9287-9293.
- Alegria HA, Wong F, Jantunen LM, Bidleman TF, Figueroa SM, et al. (2008) Organochlorine pesticides and PCBS in air of southern Mexico (2002-2004). Atmos Environ 42(38): 8810-8818.
- Andli BRRC, Bucheli TD, Ammann S, Desaules A, Keller A, et al. (2008) Lipid containing semiperme-able membrane devices for monitoring organic contaminants in water. J Environ Monit 101278–1286.
- Annual EU-wide Pesticide Residues Monitoring Reports (2002) Monitoring of pesticide residues in products of plant origin in the European Union, Norway, Iceland and Liechtenstein. Report Summary.
- Arrebola, FJ, Egea Gonzalez FJ, Moreno M, Fernandez GA, Hernandez- Torres ME, et al. (2001) Evaluation of endosulfan residues in vegetables grown in greenhouses. Pest Manage Sci 57(7): 645-52.
- Bennett P working up the toxic patient.
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, et al. (2004) Exposure of woman to organochlorine pesticides in southern spain. Environ Res 96: 34-40.
- Bouwman H, Coopan RM, Reinecke AJ, Becker PJ (1990) Levels of DDT and metabolites in breast milk from KwaZulu mothers after DDT application for malaria control. Bull World Health Organ 68(6): 761-768.
- Carreno J, Rivas A, Granada A, Lopez-Espinosa MJ, Mariscal M, et al. (2007) Exposure of young men to organochlorine pesticides in southern Spain. Environ Res 103(1): 55-61.
- Centres for disease control and prevention: department of health and human services, fourth national report on human exposure to environmental chemicals.
- Cerrillo I, Granada A, Lopez EMJ, Olmos B, Jimenez M, et al. (2005) Endosulfan and its metabolites in fertile women, placenta, cord blood and human milk. Environ Res 98(2): 233-239.
- Cerrillo I, Olea-Serrano MF, Ibarluzea J, Expoxito J, Torne P, et al. (2006) Environmental and lifestyle factors for organochlorine exposure among women living in Southern Spain. Chemosphere 62(11): 1917-1924.
- Environmental working group, BPA and other cord blood pollutants, 2016.
- Fernandez MF, Rivas A, Olea-Serrano F, Cerrillo I, Molina JM, et al. (2004). Assessment of total effective xenoestrogen burden in adipose tissue and identification of chemicals responsible for the combined estrogenic effect. Anal Bioanal Chem 379(1): 163-170.
- Garcia-Repetto R, Repetto M (1997) HCH and DDT residues in drinking water from the South of Spain, 1991-1994. Bull Environ Contam Toxicol 59(6): 875-881.
- Goerke H, Weber K, Bornemann H, Ramdohr S, Plötz J (2004) Increasing levels and biomagnification of persistent organic pollutants (POPs) in Antarctic biota. Mar Pollut Bull 48(3-4): 295-302.
- Genuis SJ, Birkholz D, Rodushkin I, Beesoon S (2011) Blood, urine, and sweat (BUS) study: monitoring and elimination of bioaccumulated toxic elements. Arch Environ Contam Toxicol 61(2): 344-357.
- Genuis SJ (2010) Elimination of persistent toxicants from the human body. Hum Exp Toxicol 30(1): 3-18.
- Genuis SJ, Beesoon S, Lobo RA, Birkholz D (2012) Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. Scientific World Journal 615068.
- Genuis SJ, Beesoon S, Birkholz D, Lobo RA (2012) Human excretion of bisphenol a: blood, urine, and sweat (BUS) Study. J Environ Public Health 185731.
- Hernandez F, Pitarch E, Serrano R, Gaspar JV, Olea N (2002) Multiresidue determination of endosulfan and metabolic derivatives inhuman adipose tissue using automated liquid chromatographic cleanup and gas chromatographic analysis. J Anal Toxicol 26(2): 94-103.
- Hou L, Zhang X, Wang D, Baccarelli A (2012) Environmental chemical exposures and human epigenetics. International Journal of Epidemiology 41(1): 79-105.
- Jandacek RJ, Genuis SJ (2013) An assessment of the intestinal lumen as a site for intervention in reducing body burdens of organochlorine compounds. Scientific World Journal 205621.
- Torres MJ, Folgoso CC, Reche FC, Velasco AR, Garcia IC, et al. (2006) Organochlorine pesticides in serum and adipose tissue of pregnant women in Southern Spain giving birth by cesarean section. Sci Total Environ 372(1): 32-38.
- Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8(6): 2265-2303.
- Sears ME, Genuis SJ (2012) Environmental determinants of chronic disease and medical approaches: recognition, avoidance, supportive therapy, and detoxification. J Environ Public Health 356798.
- Tanabe S, Subramanian A (2005) Bioindicators suitable for POPs monitoring in developing countries. Kyoto University Press, Japan.
- Stockholm Convention: Protecting health and the environment from persistent organic pollutants. United Nations Environment Programme.
- Uversky VN, Li J, Bower K, Fink AL (2002) Synergistic effects of pesticides and metals on the fibrillation of a-synuclein: implications of parkinson’s disease. Neurotoxicology 23(4-5): 527-536.
- Verla AW, Ngozi VE (2017) Risk associated with heavy metal concentrations in children playground soils of owerri metropolis, Imo state, Nigeria. World News of Natural Science 49-69.
- Versky VNU, Li J, Bower K, Fink AL (2002) Synergistic effects of pesticides and metals on the fibrillation of a-synuclein: implications for parkinson’s disease. Neurotoxicology 23(4-5): 527-536.
- Wells DE, Hess P (2000) Determination and evaluation of chlorinated biphenyls. In: Barceló E (Eds) Sample handling and trace analysis of pollutants, techniques, applications and quality assurance. Elsevier 239- 285.
- Wells DE, Hess P (2000) Separation, clean-up and recoveries of persistant trace organic contaminants from soils, sediment and biological matrices. In: Barceló E (Eds) Sample handling and trace analysis of pollutants, techniques, applications and quality assurance. Elsevier 73-116.
- Luz (2014) Dynamic association with donor cell filopodia and lipidmodification are essential features of Wnt8a during patterning of the zebrafish neuroectoderm.
Article Type
Research Article
Publication history
Received Date: May 11, 2022
Published: June 13, 2022
Address for correspondence
Verla Andrew Wirnkor, Department of Chemistry, Imo State University, Nigeria
Copyright
©2022 Open Access Journal of Biomedical Science, All rights reserved. No part of this content may be reproduced or transmitted in any form or by any means as per the standard guidelines of fair use. Open Access Journal of Biomedical Science is licensed under a Creative Commons Attribution 4.0 International License
How to cite this article
Verla Andrew W, Muna Stella E, Chigbo Medo A, Verla Evelyn N, Ali B, Enyoh Christian E, et al. African Children in Danger from High Levels Organochlorine Pesticides in Blood and Urine Samples. 2022- 4(3) OAJBS.ID.000457.
Figure 1: Elimination ratios for OCPs.
Figure 2: Principal component plot in rotated space diagram for OCPs.
Figure 3: Regression plots for OCPs in blood and urine of children.
Table 1: Concentration of OCPs in whole blood and morning urine samples of children.
Table 2: Correlation coefficient matrix for OCPs in blood and urine.
Table 3: Correlation coefficient matrix for OCPs in blood and urine.
Table 4: Ratios OCPs concentrations in blood and urine.